Microarray patch resources
PATH is advancing the microarray patch (MAP) delivery technology platform for high priority vaccines and essential medicines.

Assortment of microarray patches from different technology developers. Photo: PATH/Patrick McKern.
1 of 7
Vaxxas Nanopatch prototype coated microarray patch. Photo: PATH/Patrick McKern.
2 of 7
Vaxxas Nanopatch prototype coated microarray patch applicator and packaging. Photo: PATH/Patrick McKern.
3 of 7
Queen’s University Belfast prototype dissolving microarray patch. Photo: PATH/Patrick McKern.
4 of 7
Georgia Tech/Micron Biomedical prototype dissolving microarray patch. Photo: PATH/Patrick McKern.
5 of 7
Georgia Tech/Micron Biomedical prototype dissolving microarray patch and packaging.
6 of 7
Acropass/Raphas commercially available dissolving microarray patch for cosmetic-use.
7 of 7Microarray Patch Center of Excellence
PATH’s Center of Excellence for Microarray Patch Technology is a multi-year initiative to mobilize efforts to accelerate the development of MAPs for critical vaccines and essential medicines and to ensure that the MAP delivery technology platform can maximize its impact by meeting global public health needs.
Together with the community of MAP developers, industry, and global health stakeholders, the PATH Center of Excellence aims to forge a future for MAPs that maximizes the potential of this innovative delivery technology platform. Connect with us via email at: MAPs@path.org
About PATH's work advancing microarray patch technology
For more than a decade, PATH has assessed and catalyzed microarray patch (MAP) transdermal technologies that have the potential to improve access to and delivery of a broad range of vaccines and essential medicines in low-resource settings. As a disruptive technology, MAPs could have a transformative impact on health.
Microarray patches, also known as microneedle patches, consist of microscopic projections that are applied to the body like a small bandage, penetrating the skin’s outermost layer to deliver a drug or vaccine to the top layers of the skin.
MAPs have shown the potential for:
- Increased thermostability: reducing cold chain burdens and facilitating access to remote areas.
- Ease-of-use: facilitating self-administration or delivery by lesser-trained health care workers.
- Needle-free delivery: eliminating the risk of needlestick injuries typically associated with needle and syringe delivery.
- Increased coverage and reduced programmatic costs: through enabling alternative delivery scenarios such as supplemental immunization activities.
Factsheet
Microarray patches for delivery of vaccines and essential medicines
PATH produced this factsheet providing a high-level overview of microarray patch technology for vaccines and essential medicines. Topics covered include: health need, advantages of MAP delivery, and current status and engagement opportunities.
Factsheet
Vaccine antigen qualities for microarray patches
To aid vaccine developers in selecting an appropriate antigen, this document overviews key antigen qualities that impact the likelihood of successful technical development for a vaccine MAP.
Packaging report
With funding from PEPFAR/USAID, PATH developed a packaging report which reviews and provides recommendations for MAP packaging, including those related to design, materials, desiccants, adhesives/seals, sterility control, usability and programmatic fit, environmental and supply chain impact, cost, and manufacturability.
Manufacturing readiness assessment
Recently, Drug Delivery and Translational Research published PATH's article, Manufacturing readiness assessment for evaluation of the microneedle array patch industry: an exploration of barriers to full-scale manufacturing.
Collaborate
Microneedle Array Patch Regulatory Working Group
This collaboration aims to inform, guide, and define the regulatory science of the MAP dosage form.
Business case analyses
Measles Rubella (MR) vaccine and contraceptive MAPs
PATH has produced two business case analyses for MR vaccine and contraceptive MAPs to inform future investment and development.
VIPS Alliance
Microarray patches prioritized through VIPS
PATH served as a key partner on the Gavi-led Vaccine Innovation Prioritisation Strategy (VIPS) along with the Bill & Melinda Gates Foundation, WHO, and UNICEF to advance vaccine product innovations with the greatest potential to meet country needs and improve immunization coverage and equity. In 2020, the VIPS process prioritized three innovations with the potential to overcome country immunization barriers to equitable access and improve the effectiveness of vaccines in low- and middle-income countries. MAPs were selected for final prioritization because they are considered "truly 'transformational' innovations that have the potential to address many immunization barriers identified by countries."
PATH’s MAP acceptability and usability research



Global map of Microarray Patch developers
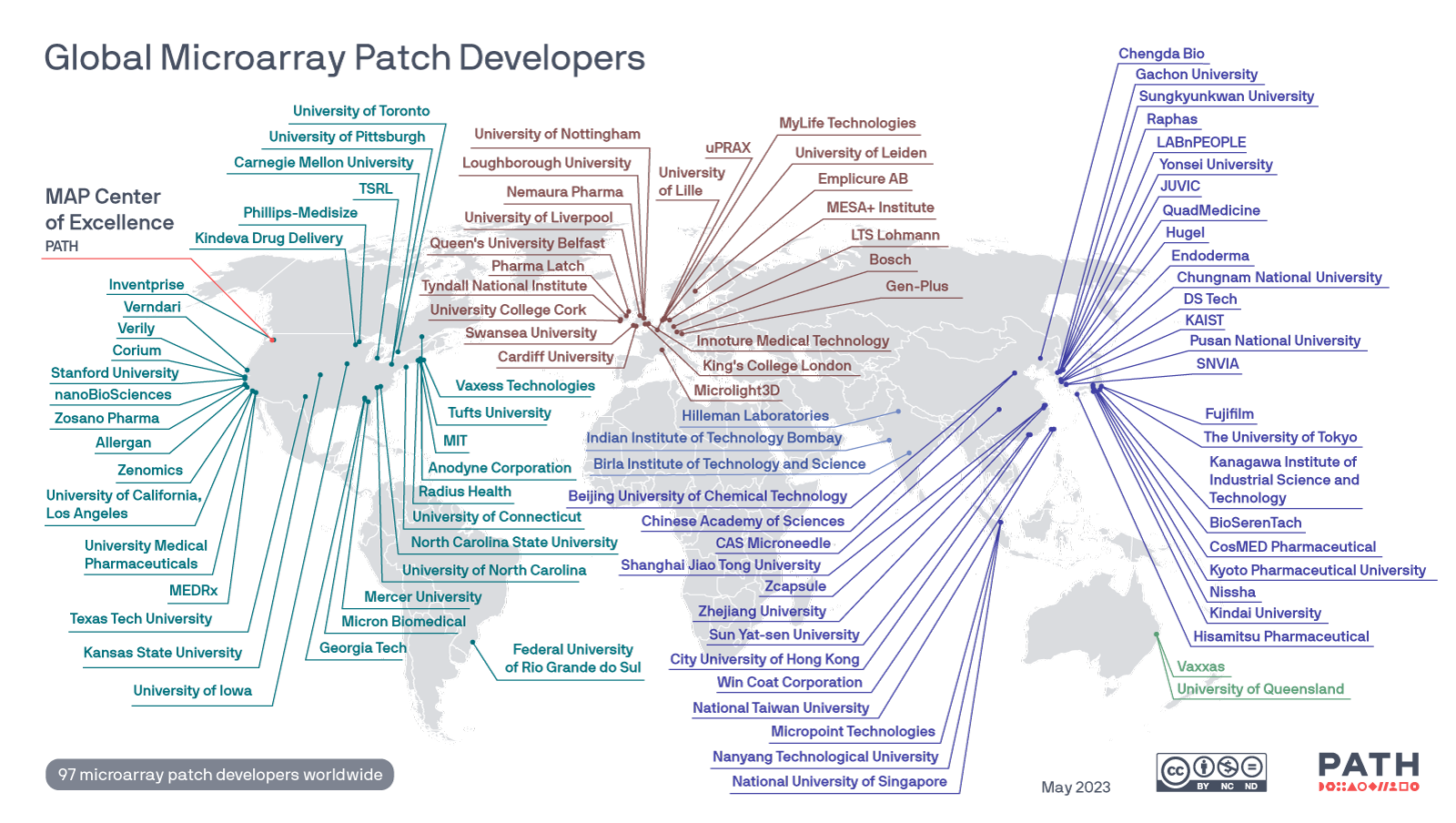
The MAP CoE created a global map tracking known MAP developers around the world and will update as needed. Image: PATH.
Versions of this map can be downloaded here.
MICROARRAY PATCH CATEGORY RESOURCES
Technology overview
- Technology update: dissolvable microneedle patches for vaccine delivery. (Rogers, 2019) Read here.
- Microneedle patches for vaccination in developing countries. (Arya, 2016) Read here.
- Potential use of microarray patches for vaccine delivery in low- and middle- income countries. (Peyraud, 2019) Read here.
- Advancing Transdermal Drug Delivery: PATH’s Microarray Patch Center of Excellence aims to accelerate transdermal patch technology for public health needs. Read here.
Immune response and skin immunology
- Characteristics of immune induction by transcutaneous vaccination using dissolving microneedle patches in mice. (Hirobi, 2021) Read here.
- Early immune responses in skin and lymph node after skin delivery of Toll-like receptor agonists in neonatal and adult pigs. (Vreman, 2021) Read here.
- Enhancing humoral immunity via sustained-release implantable microneedle patch vaccination. (Boopathy, 2019) Read here.
- Dissolvable carboxymethyl cellulose/polyvinylpyrrolidone microneedle arrays for transdermal delivery of Amphotericin B to treat cutaneous leishmaniasis. (Zare MR, 2021) Read here.
- Langerin negative dendritic cells promote potent CD8+ T-cell priming by skin delivery of live adenovirus vaccine microneedle arrays. (Bachy, 2013) Read here.
- Skin immunisation activates an innate lymphoid cell-monocyte axis regulating CD8+ effector recruitment to mucosal tissues. (Zaric, 2019) Read here.
- Skin vaccination with live virus vectored microneedle arrays induce long lived CD8(+) T cell memory. (Becker, 2015) Read here.
Usability and end-user studies
- End-user acceptability study of the nanopatchTM; a microarray patch (MAP) for child immunization in low and middle-income countries. (Guillermet, 2019) Read here.
HEALTH AREA RESOURCES
Contraception
- Long-acting reversible contraception by effervescent microneedle patch. (Li, 2019) Read here.
- Microarray patches: Breaking down the barriers to contraceptive care and HIV prevention for women across the globe. (Paredes, 2021) Read here.
- Silk Fibroin Microneedle Patches for the Sustained Release of Levonorgestrel. (Yavuz, 2020) Read here.
COVID-19
- Dissolvable microneedle patches to enable increased access to vaccines against SARS-CoV-2 and future pandemic outbreaks (O'Shea, 2021). Read here.
- Induction of humoral and cellular immunity by intradermal delivery of SARS-CoV-2 nucleocapsid protein using dissolvable microneedles. (Kuwentrai, 2021) Read here.
- Microarray patches enable the development of skin-targeted vaccines against COVID-19. (Korkmaz, 2021) Read here.
Dengue virus
- A chimeric dengue virus vaccine candidate delivered by high density microarray patches protects against infection in mice. (Choo, 2021). Read here.
- Efficient Delivery of Dengue Virus Subunit Vaccines to the Skin by Microprojection Arrays. (Muller, 2019) Read here.
Diabetes
- Fabrication of rapidly separable microneedles for transdermal delivery of metformin on diabetic rats. (Liu, 2021) Read here.
Ebola
- Intradermal immunization by Ebola virus GP subunit vaccines using microneedle patches protects mice against lethal EBOV challenge. (Liu, 2018) Read here.
Hepatitis B
- Feasibility of hepatitis B vaccination by microneedle patch: Cellular and humoral immunity studies in rhesus macaques. (Choi, 2019). Read here.
- Skin immunization with third-generation hepatitis B surface antigen using microneedles. (Nguyen, 2019) Read here.
HIV and HIV PrEP
- Acceptability of Microarray Patches for Delivery of HIV Pre-exposure Prophylaxis (PrEP) Among Women and Health Care Providers in South Africa. (PATH, 2016) Read here.
- Design, formulation and evaluation of novel dissolving microarray patches containing a long-acting rilpivirine nanosuspension. (Mc Crudden, 2018) Read here.
- Etravirine-loaded dissolving microneedle arrays for long-acting delivery. (Rojekar, 2021) Read here.
- Long-lived tissue resident HIV-1 specific memory CD8+ T cells are generated by skin immunization with live virus vectored microneedle arrays. (Zaric, 2017) Read here.
- Microarray patches: Breaking down the barriers to contraceptive care and HIV prevention for women across the globe. (Paredes, 2021) Read here.
- Modelling the intradermal delivery of microneedle array patches for long-acting antiretrovirals using PBPK. (Rajoli, 2019) Read here.
- Vaginal Applicator Development for Delivery of Microarray Patches Containing Rilpivirine for HIV Pre-exposure Prophylaxis. (PATH, 2016) Read here
Human papillomavirus
- Immune response and reactogenicity of an unadjuvanted intradermally delivered human papillomavirus vaccine using a first generation Nanopatch™ in rhesus macaques: An exploratory, pre-clinical feasibility assessment. (Meyer, 2019) Read here.
- Prophylactic HPV vaccine microarray patch: Target product profile. Draft. (PATH, 2020) Read here.
Influenza
- An economic model assessing the value of microneedle patch delivery of the seasonal influenza vaccine. (Lee, 2015) Read here.
- Clinical study and stability assessment of a novel transcutaneous influenza vaccination using a dissolving microneedle patch.(Hirobe,
2015) Read here. - Formulation approach that enables the coating of a stable influenza vaccine on a transdermal microneedle patch. (Ameri, 2021) Read here.
- Opportunities and challenges in delivering influenza vaccine by microneedle patch. (Jacoby, 2015) Read here.
- Safety, tolerability, acceptability and immunogenicity of an influenza vaccine delivered to human skin by a novel high-density microprojection array patch (Nanopatch™). (Fernando, 2018) Read here.
- Safety, tolerability, and immunogenicity of influenza vaccination with a high-density microarray patch: Results from a randomized, controlled phase I clinical trial. (Forster, 2020) Read here.
- The Safety, Immunogenicity and Acceptability of Inactivated Influenza Vaccine Delivered by Microneedle Patch: a Randomized, Partially Blind, Placebo-Controlled Phase 1 Trial. (Rouphael, 2017) Read here.
Malaria
- Artemether and lumefantrine dissolving microneedle patches with improved pharmacokinetic performance and antimalarial efficacy in mice infected with Plasmodium yoelii. ( Volpe-Zanutto, 2021) Read here.
- Assessing the Technical and Programmatic Feasibility of a Microarray Patch for Intradermal Delivery of Primaquine to Treat P. vivax. (PATH, 2017) Read here.
Measles Rubella
- A Microneedle Patch for Measles and Rubella Vaccination Is Immunogenic and Protective in Infant Rhesus Macaques. (Joyce, 2018) Read here.
- A microneedle patch for measles and rubella vaccination: a game changer for achieving elimination. (Prausnitz, 2020) Read here.
- Formulation development and improved stability of a combination measles and rubella live-viral vaccine dried for use in the nanopatch microneedle delivery system. Read here.
- Measles and rubella microarray array patches to increase vaccination coverage and achieve measles and rubella elimination in Africa. (Moss, 2019) Read here.
- Measles-rubella microarray patch (MR–MAP) target product profile. (WHO, UNICEF 2019) Read here.
Neonatal and infant health
- Transdermal delivery of gentamicin using dissolving microneedle arrays for potential neonatal sepsis. (González-Vázquez, 2017) Read here.
Polio
- High-density microprojection array delivery to rat skin of low doses of trivalent inactivated poliovirus vaccine elicits potent neutralising antibody responses. (Muller, 2017) Read here.
- Inactivated polio vaccination using a microneedle patch is immunogenic in the rhesus macaque. (Edens, 2015) Read here.
- Skin delivery of trivalent Sabin inactivated poliovirus vaccine using dissolvable microneedle patches induces neutralizing antibodies. (Donadei, 2019) Read here.
Rabies
- Rabies vaccine microarray patch: Target product profile. Draft. (PATH, 2020) Read here.
PATH co-chairs the Delivery Technologies Working Group within WHO’s Immunization Practices Advisory Committee (IPAC). Its mission is to maximize impact of immunization products for public-sector use, including combination vaccine/device products such as MAPs and other alternative delivery technologies. A list of resources related to vaccine delivery technologies, as well as materials developed by the Delivery Technologies Working Group, are available through TechNet-21, a global network of immunization professionals.
Meet the MAP Center of Excellence director
-
![]()
Courtney Jarrahian
Global Program Leader


