
Strengthening R&D and regulatory systems
How PATH works with change-makers to smooth regulatory pathways for more equitable health systems
At PATH, we believe everyone should have access to lifesaving health technologies and interventions, no matter where they live.
Unfortunately, far too many people living in low- and middle-income countries lack access to essential health products, resulting in more illness and death. Inequities in access are particularly stark during crises like COVID-19, when, for example, countries in Africa had to wait months for vaccines and treatments already accessible in high-income countries to stem the pandemic.
In Africa, lack of access stems from a number of challenges with the R&D ecosystem, including limited research infrastructure and complicated regulatory systems across the continent. However, much progress has been made since 2020.
By collaborating with regional bodies, governments, and civil society organizations, we aim to foster a more inclusive R&D environment—from early research to product development and manufacturing done on the continent—to address the health challenges countries face today and the future threats that may emerge.
As a trusted partner to several regional bodies, we have helped influence improvements to the R&D and regulatory ecosystem post-COVID, such as the ratification of the African Medicines Agency and the creation of the Platform for Harmonized African Health Manufacturing. We are also exploring how integrating Artificial Intelligence (AI) into health care can enhance clinical research, diagnostics, treatment, and data-driven decision-making, improving access to quality care both in Africa and globally.

This interactive dashboard catalogues information about government and private entity commitments worldwide to improving the end-to-end R&D ecosystem in Africa.
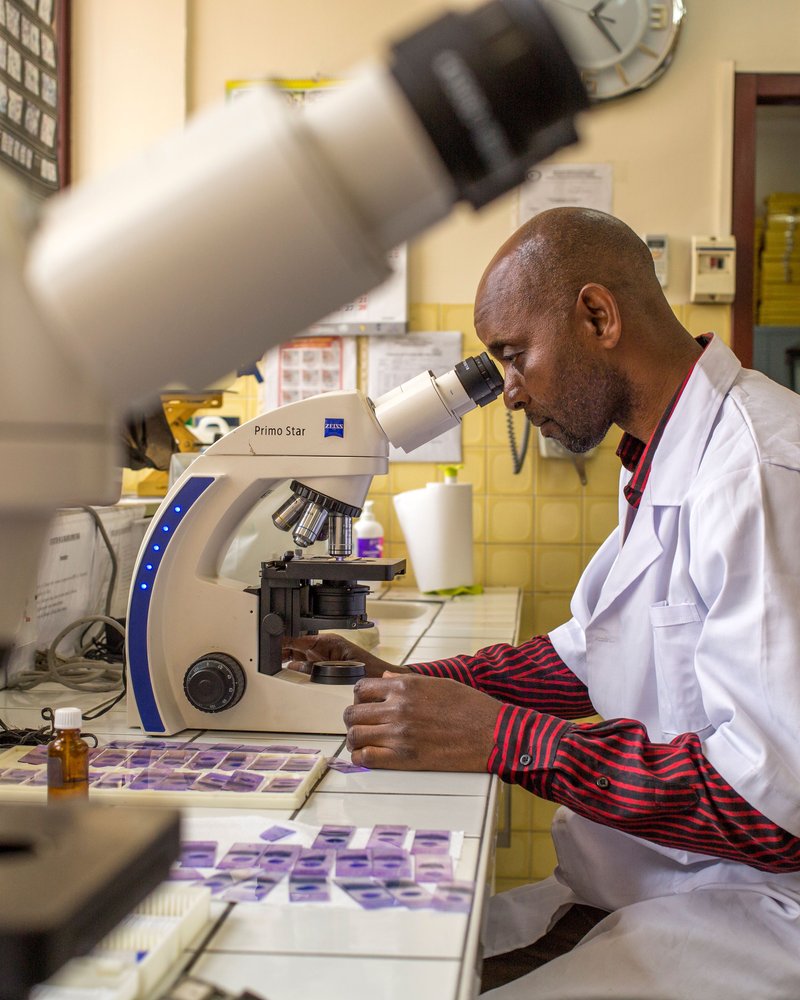
PATH supports countries in ratifying the African Medicines Agency Treaty to expand access to safe and high-quality medical products across the continent.

A PATH-led study to assess the current and planned state of vaccine manufacturing in Africa and provides insights into what’s needed to develop a robust and sustainable vaccine manufacturing ecosystem.

Prepared by the staff of the African Union Development Agency (AUDA-NEPAD) and PATH, this training guide on regulatory systems strengthening in Africa is designed for National Medicines Regulatory Authorities, Regional Economic Communities, and all other stakeholders able to influence and support regulatory systems at the country, regional, or continental level.


