World’s first malaria vaccine shows dramatic impact on child deaths
Pilot introductions of the RTS,S/AS01 malaria vaccine in Ghana, Kenya, and Malawi concluded in 2023, yielding exciting data about the impact of the vaccine in routine immunization. The pilot evaluations, 46 months in length, showed a 13 percent drop in deaths from all causes (excluding accident and injury) among children age-eligible to receive the vaccine and a 22 percent decrease in severe malaria hospitalizations. These and other data were shared at the annual meeting of the American Society of Tropical Medicine and Hygiene in October 2023 and are expected to be published in 2024.

Alice Musimbi received her first dose of RTS,S following an expansion of Kenya’s pilot introduction in 2023. PATH and GSK have spent 20+ years developing the vaccine—the world’s first against malaria and against any parasite. Photo: PATH/Ruth Wanjala
1 of 2 2 of 2
2 of 2
Just one month later, on November 22, a shipment of more than 330,000 doses of RTS,S from GSK arrived in Cameroon—the first such shipment to a non-pilot country. Excitement was high, as Cameroon was expected to begin vaccinations early in 2024. Nikolaj Gilbert, President and CEO of PATH, acknowledged the significance of the moment to PATH, saying: “I am thrilled that the RTS,S vaccine, which is the result of so many years of work by PATH, GSK, and African partners, has arrived in Cameroon and will soon reach even more children at risk of malaria.” He went on to acknowledge the strenuous efforts of global partners to accelerate access to RTS,S, saying, “All of us at PATH appreciate the efforts by Gavi, UNICEF, and WHO to accelerate access to this lifesaving vaccine.”
Since 2001, PATH has been instrumental in the development and subsequent pilot introductions of this complementary malaria intervention. PATH continues to support the ministries of health in Ghana, Kenya, and Malawi in their ongoing implementation of RTS,S and is assisting other countries as they prepare to introduce and roll out approved malaria vaccines.
This work advanced the Preparing for and responding to emerging health threats (P1) and Improving lives with science and technology (P2) strategic priorities.
A new vaccine could stop meningococcal meningitis epidemics in Africa
In July 2023, the World Health Organization (WHO) prequalified the MenFive® vaccine—the first conjugate vaccine to protect against the five predominant causes of meningococcal meningitis in the African meningitis belt (serogroups A, C, W, Y, and X). Developed over a decade-plus period by Serum Institute of India Pvt. Ltd. (SIIPL) and PATH, with funding from the United Kingdom’s Foreign, Commonwealth & Development Office, MenFive was designed to eliminate annual meningococcal meningitis outbreaks and epidemics in the African meningitis belt—a string of 26 countries from Senegal in the west to Ethiopia in the east—which faces the greatest meningitis burden on Earth.

The 2010 introduction of MenAfriVac (pictured) virtually eliminated meningitis A for 400 million people in the African meningitis belt. The new MenFive vaccine could end outbreaks of all five major types (A, C, W, Y, X). Photo: PATH/Gabe Bienczycki
1 of 2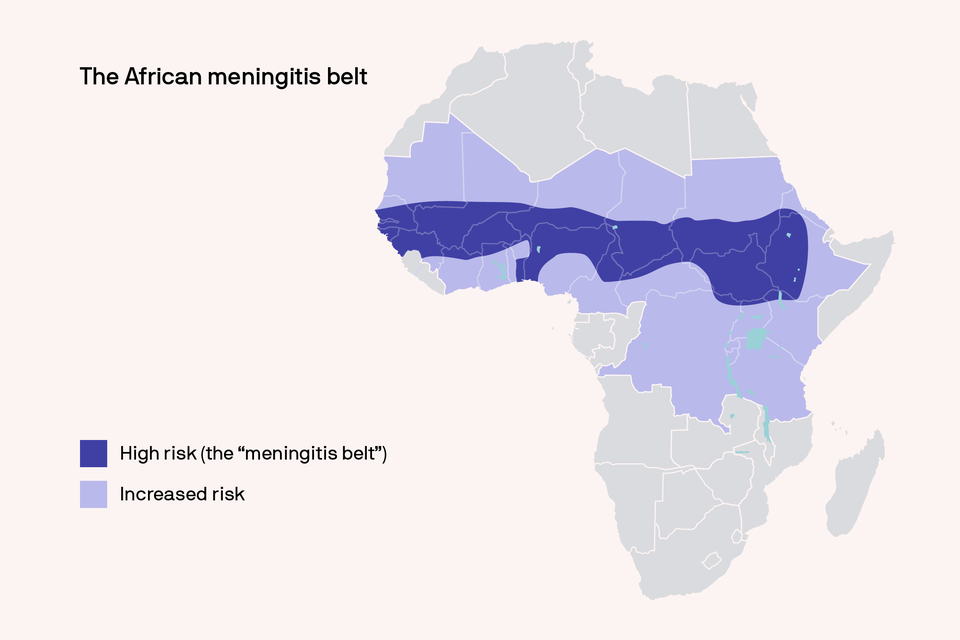 2 of 2
2 of 2
Historically, emergency vaccination campaigns have been the only recourse for African countries suffering meningococcal epidemics. However, the polysaccharide vaccines used in these reactive campaigns have limitations that can prevent sustainable impact. As a conjugate vaccine, MenFive overcomes these limitations by protecting children younger than 2 years of age and providing herd protection to the unvaccinated. It was also designed to be more affordable than existing meningococcal conjugate vaccines. Importantly, MenFive is the only vaccine that prevents meningitis caused by serogroup X, a pathogen increasingly implicated in African meningitis outbreaks.
MenFive builds on the legacy of MenAfriVac®, SIIPL’s groundbreaking vaccine—developed in partnership with PATH and WHO—that eliminated meningitis A outbreaks in the African meningitis belt following its 2010 introduction. Approved by WHO for individuals 1 to 85 years of age, MenFive was used for the first time in March 2024 to tackle a meningitis C outbreak in Nigeria. Discussions are underway about ways to incorporate MenFive into mass vaccination campaigns and routine immunization schedules.
This work advanced the Preparing for and responding to emerging health threats (P1) and Improving lives with science and technology (P2) strategic priorities.
PATH team files patent for groundbreaking heat-stable insulin
Insulin is a lifesaving medication for people with diabetes. Though WHO considers insulin an essential medicine, only one in two diabetes patients worldwide can access it. Barriers to insulin access include the high cost of the medicine, the difficulties of managing frequent injections, and the need for a complex cold chain infrastructure to keep the drug at the right temperature during transport and storage.

Worldwide, more than 400 million people are living with diabetes but half of those are unable to access insulin because of cost or other barriers. PATH’s new heat-stable insulin formulation could improve access for millions. Photo: PATH/Patrick McKern
1 of 3
A close up of PATH's new heat-stable insulin formulation in blister packs. Photo: PATH/Patrick McKern.
2 of 3 3 of 3
3 of 3
Scientists from PATH’s formulation team have developed a heat-stable insulin, which has the potential to improve accessibility and adherence for millions of people around the world. In 2023, the team demonstrated their novel formulation achieved lower and sustained glucose levels in diabetic rodents for longer than 8 hours and held up better than liquid insulin at high humidity and temperatures. They will continue testing its stability and effectiveness and are working toward a product that is easy and inexpensive to transport, store, and administer.
“One hundred years after its discovery, insulin is still inaccessible to millions of people who need it,” said Dr. Manjari Lal, Research and Development Advisor for PATH’s Medical Devices and Health Technologies program. “Our aim is to accelerate access to this lifesaving medicine through improvements in stability and storage conditions.”
This work advanced the Improving lives with science and technology (P2) strategic priority.
Increasing access to fortified rice
PATH is a pioneer of rice fortification technology and has helped many countries scale up rice fortification programs to meet localized micronutrient needs. To date, these efforts have enhanced nutrition for approximately 800 million people around the world, but our work is far from over.

Fortified rice being cooked for a school lunch program in India. PATH provides technical expertise for scale-up of fortified rice, supply chain optimization, quality control, social and behavior change, evidence generation, and more. Photo: PATH
1 of 3
A package of BB Royal Fortified private label fortified rice. Photo: BB Royal
2 of 3 3 of 3
3 of 3
PATH teams are still strengthening the capacities of rice millers, fortified rice kernel manufacturers, food safety officials, and other government officials to increase access to this affordable and impactful nutrition intervention. During the Micronutrient Forum’s 6th Global Conference in 2023, PATH India launched a global rice fortification resource hub called FortiRice. This information platform is helping to create an ecosystem that promotes local production of fortified rice and its integration into food systems.
In addition to the launch of FortiRice, PATH provided key technical support to India’s BigBasket online supermarket toward successful launch of its BB Royal Fortified private label assortment. The introduction of BB Royal fortified rice has enabled access to fortified rice in the open market and is a significant milestone in addressing the issues of anemia and malnutrition, which are not confined solely to marginalized populations.
This work advanced the Improving lives with science and technology (P2) and Increasing health system capacity and resilience (P3) strategic priorities.
Contraceptive self-injection gains momentum
In 2023, PATH launched a new phase of the Access Collaborative to advance introduction and scale-up of the contraceptive subcutaneous DMPA (DMPA-SC), including through self-injection. The Access Collaborative provides data-driven technical assistance, coordination, resources, and tools to ensure that women and girls have increased access to DMPA-SC and self-injection as part of an expanded range of contraceptive methods, delivered through informed choice programming.
Since 2017, the Access Collaborative has supported 66 countries with technical assistance, resources, and skills-building. These efforts have contributed to increased uptake, with more than 1.7 million client self-injection visits in the 14 countries that share self-injection data with the Access Collaborative. Formerly known as the DMPA-SC Access Collaborative, the new Injectables Access Collaborative includes an expanded focus on global market management and analytics for DMPA-SC as part of the broader injectable contraceptive market. This initiative is led by PATH in partnership with the Clinton Health Access Initiative, inSupply Health, Jhpiego, and JSI.
In Uganda, a woman self-injects the contraceptive DMPA-SC (Pfizer’s Sayana® Press) using a prefilled BD UnijectTM injection system, which was originally developed by PATH. Photo: PATH/Gabe Bienczycki
1 of 3 2 of 3
2 of 3
 3 of 3
3 of 3
By putting the power of protection in women’s hands, self-injection with DMPA-SC has the potential to reduce access-related barriers, increase contraceptive continuation rates, and enhance women’s autonomy. Monica Mutesa, Technical Advisor, Zambia, recently shared that one area in Zambia reported high rates of clients self-injecting DMPA-SC, and when she followed up with the local health care team to learn more, the team highlighted the dangers women face when traveling to clinics. Particularly during the rainy season, wild animals pose a threat along these routes, which women often travel by foot. Self-injection emerged as a solution, significantly reducing the number of clinic visits required. Having access to a self-injectable contraceptive option can not only save women time and money but also enable them to use family planning safely at their convenience.
This work advanced the Improving lives with science and technology (P2) and Increasing health system capacity and resilience (P3) strategic priorities.
PATH advances people-centered primary health care to make good health more accessible for everyone—especially those living with (or at risk of) infectious or noncommunicable diseases.
From policy advocacy and financing to technical assistance and workforce training, PATH partners with governments and local nongovernmental organizations to enhance health system capacity and resilience.
2023 PATH annual report
Annual report home | PATH Strategy 2025 | Product development and access | Health and disease management | Health systems strengthening | Financial summary | Leadership | Supporters
