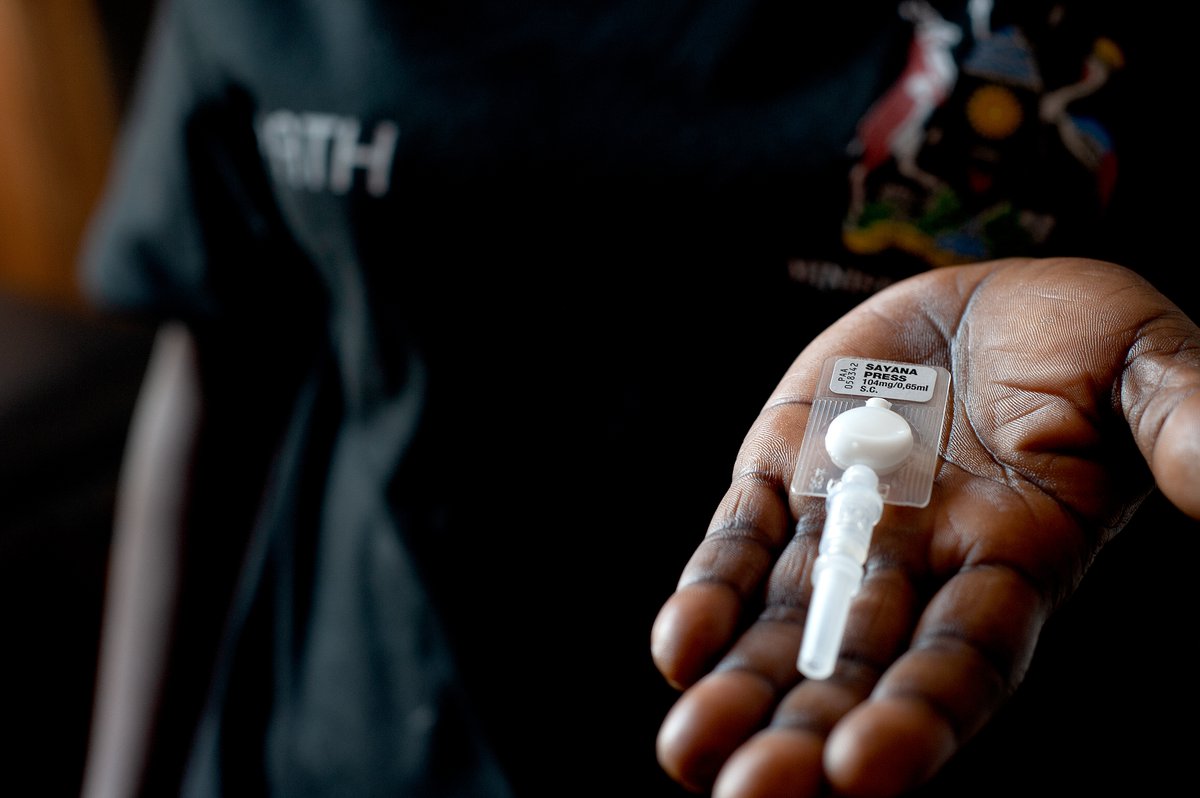
DMPA-SC self-injection resources
A new practice in family planning is revolutionizing contraceptive access and use. Self-injected contraception is now an option with the innovative, easy-to-use injectable DMPA-SC.
Self-injection with DMPA-SC (brand name Sayana® Press) has the potential to reduce access-related barriers for women, increase contraceptive continuation rates, and enhance women’s autonomy. There is clear evidence that women—including women in low-resource settings—can self-administer DMPA-SC safely and effectively, and that they like doing so.
An increasing number of countries worldwide are adding DMPA-SC to their contraceptive method mix and including self-injection as one option women can choose. Senegal and Uganda plan to scale up self-injection, and the Democratic Republic of the Congo, Ghana, Malawi, and Nigeria are in early stages of introducing the practice. And PATH’s Self-Injection Best Practices initiative in Uganda is identifying self-injection program approaches that work, informing policy and practice worldwide.
For more information, visit www.path.org/dmpa-sc, sign up for our newsletter, or email FPoptions@path.org.
Resources
Overview and training resources
- Self-injected subcutaneous DMPA: A new frontier in advancing contraceptive access and use for women
- Self-injection: Empowering Women Who Want to “Stick” with Contraception
- Self-Injection Best Practices web page
- DMPA-SC training materials for providers and self-injection clients
Research briefs
- DMPA-SC Self-injection Best Practices project brief
- Self-injection feasibility and acceptability brief
- DMPA-SC self-injection continuation research brief
*DMPA-SC: Subcutaneous depot medroxyprogesterone acetate.
Sayana Press is a registered trademark of Pfizer Inc. Uniject is a trademark of BD.

