Developing the world’s first malaria vaccine (RTS,S)
Learn how PATH and partners advanced this lifesaving innovation for African children.
Product development
Malaria
Research and development
Vaccines
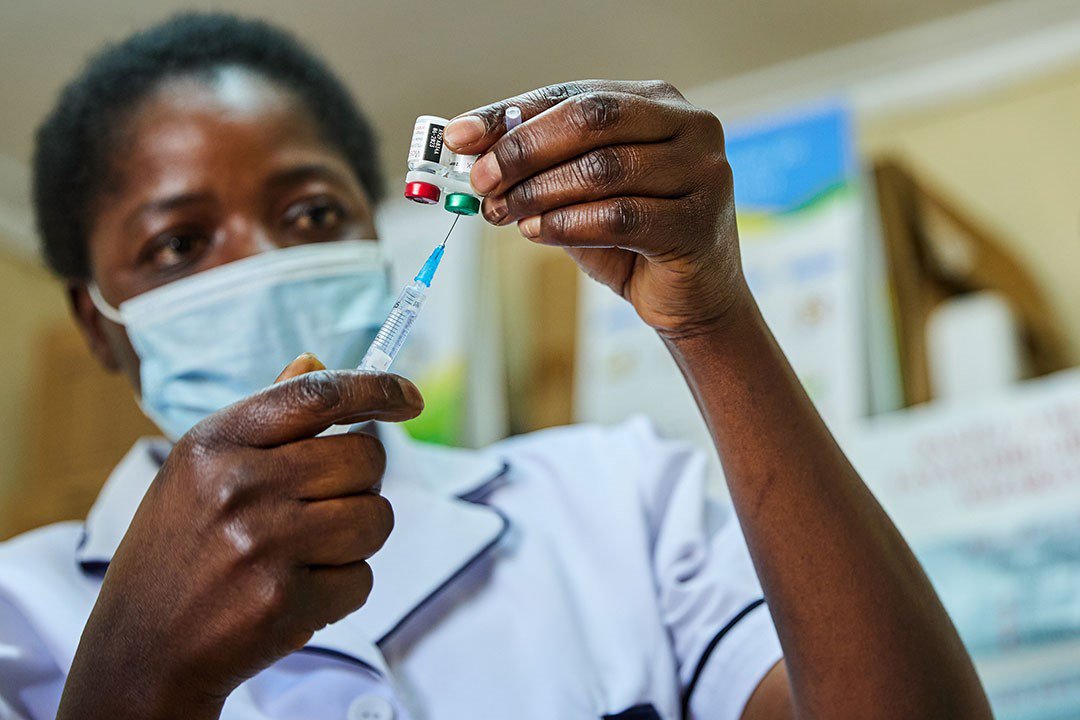
The challenge
Malaria kills more than 600,000 people a year worldwide and causes illness in more than 200 million more. The majority of deaths occur among young children living in sub-Saharan Africa.
Malaria negatively affects all stages of human development. It contributes to anemia in pregnant women and low birth weight, premature birth, and infant mortality in their children. Even when children survive malaria, their mental and physical development is often negatively affected. Malaria can have a debilitating effect on adults, as well, often removing them from the workforce for days or weeks at a time.
Interventions such as vector control, chemoprevention, and prompt diagnosis and treatment have helped to reduce malaria deaths significantly over the past two decades, but the need for new and improved tools, including the accelerated development of a malaria vaccine, has long been clear.
“Parasites of the type that cause malaria are complex [and] adaptable and have evolved to move seamlessly through the human and mosquito populations in most of Africa,” according to Ashley Birkett, PhD, who is global head of Parasitic and Bacterial Diseases in PATH’s Center for Vaccine Innovation and Access (CVIA) and an expert in malaria vaccine development. “Therefore, setting out to develop a vaccine for malaria wasn’t going to be an easy process. In addition to the significant biological challenges, there was no strong business case for major pharmaceutical companies to invest in a malaria vaccine. The disease wasn’t a priority for high-income markets.”
The solution
Today, two safe and effective malaria vaccines have been approved by the World Health Organization (WHO) and are becoming available to help close the gap left by other interventions: RTS,S/AS01 RTS,S) and R21/Matrix-M (R21). PATH played a key role in developing and introducing RTS,S—the world’s first malaria vaccine and the world’s first vaccine against a parasite in humans.
RTS,S was created in 1987 by scientists at GSK. Early clinical development was conducted in collaboration with the Walter Reed Army Institute for Research. The vaccine is designed to prevent the parasite from infecting a human host’s liver, where it can mature, multiply, reenter the bloodstream, and infect red blood cells.
According to Dr. Birkett, “the invention of the RTS,S vaccine is due to the creativity of talented scientists like Joe Cohen at GSK. His particular expertise solved this scientific problem in a singular manner.”
In 2001, GSK and PATH’s Malaria Vaccine Initiative entered into a public-private partnership to develop RTS,S for young children living in malaria-endemic regions in sub-Saharan Africa. Supported by the Bill & Melinda Gates Foundation, PATH provided technical and project management support for RTS,S development, working with GSK and African research centers to generate the data required for policy decisions regarding the potential use of the vaccine in young African children.
During this period, RTS,S was rigorously tested through a series of clinical trials.¹˒² PATH supported the management of Phase 2 studies of RTS,S in young African children, which in 2004 demonstrated efficacy in children under five years of age. These and other Phase 2 studies yielded sufficiently positive results to warrant advancement to Phase 3, including the pivotal Phase 3 efficacy and safety trial, which ran from 2009 to 2014.
This trial involved 15,459 infants and young children and was conducted by 11 clinical research centers in seven African countries (Burkina Faso, Gabon, Ghana, Kenya, Malawi, Mozambique, and Tanzania).³ Together, scientists at the research centers, GSK, and PATH formed the Clinical Trials Partnership Committee, led by African research scientists, which oversaw the Phase 3 trial and authored the key publications on the trial results.
The Phase 3 trial demonstrated that malaria episodes were reduced by more than half during the first year after vaccination. Over a four-year period, among children who received all four doses, the vaccine reduced malaria episodes by about 40 percent and severe malaria cases by about 30 percent.³ The study also showed that at the trial site with the highest disease burden, more than 6,500 clinical malaria episodes were averted for every 1,000 children who were fully vaccinated.
In an extension of the Phase 3 efficacy and safety trial, a study of the longer-term impact of the vaccine, with a focus on severe malaria, was completed in December 2016. This study followed children who had participated in the trial at 3 of the 11 research centers for an additional three years, for a total of seven years of follow-up. Results showed that the incidence of severe malaria decreased as children got older, regardless of whether children received the vaccine; there was no evidence of rebound of severe malaria following the recommended four doses.⁴˒⁵
In January 2016, WHO accepted the advice of its global advisory bodies on immunization and malaria to recommend pilot implementation of the RTS,S malaria vaccine in three to five settings of moderate to high malaria transmission in sub-Saharan Africa.² This led to creation of the RTS,S Malaria Vaccine Implementation Programme, a WHO-coordinated pilot program designed to evaluate the feasibility, safety, and impact of the vaccine in real-life childhood vaccination settings.
Pilot vaccinations began in Ghana, Kenya, and Malawi in 2019. The three countries led the rollout of RTS,S in selected areas, with funding and technical support from the Malaria Vaccine Implementation Programme’s global partners, including PATH. The pilots were financed through a collaboration involving Gavi, the Vaccine Alliance; the Global Fund to Fight AIDS, Tuberculosis and Malaria; and Unitaid.
“Two years into the pilot introduction, it was already clear that the RTS,S malaria vaccine was having an impact,” said John Bawa, director of the CVIA’s malaria vaccine implementation. “For a long time, advocates and experts had been waiting for an additional tool to add to the malaria toolkit—something that would help protect more children across Africa where the burden of malaria is greatest. It was so gratifying to support the pilot countries to access this new tool, and the results are extremely promising.”
On October 6, 2021, WHO recommended RTS,S for widespread use in young children based upon the evidence generated by the pilots, as well as data from studies conducted after 2016 (see further below). In 2022, the vaccine received WHO prequalification, the process by which WHO attests to a vaccine’s safety, effectiveness, and quality.
“WHO prequalification is the critical step toward expanding access to the vaccine,” said Deborah Atherly, global head of the CVIA’s Policy, Access, and Introduction. “Prequalification allows UNICEF to purchase doses for use in [United Nation] Member States, and it allows Gavi, the Vaccine Alliance to provide financial assistance to eligible countries for vaccine introduction. This milestone really helped make this lifesaving vaccine accessible for more children in more communities.”
In late 2022 and early 2023, the remaining pilot areas of Ghana, Kenya, and Malawi began receiving RTS,S as part of an initial expansion of vaccination. This expansion was made possible by a grant to PATH from the US-based Open Philanthropy to support scale-up of RTS,S use through 2023, using vaccine doses donated by GSK and working in collaboration with WHO and other in-country partners.

Alice Musimbi received her first RTS,S dose after Kenya expanded vaccine use in pilot areas in early 2023. Pilots showed 13 percent fewer deaths among children eligible to receive the vaccine. Alice pictured with her mother. Photo: PATH/Ruth Wanjala.
Why was PATH chosen to do this work?
By the late 1990s, it was becoming clear that the private sector alone could not provide the products needed to address neglected tropical diseases.
“With neglected tropical diseases like malaria, the populations at risk are predominantly in low-income countries. Private-sector institutions would not be able to recoup research and development costs through sales to these countries, so it was almost impossible to develop these products which were so badly needed without an innovative and collaborative approach,” explained Dr. Birkett.
The product development partnership model emerged to address this challenge. Several such partnerships were established in the late 1990s and early 2000s to help potentially lifesaving medical products overcome the lack of funding for late-stage development, a phase that requires significant financial resources.
PATH was already known for its role as a trusted, reliable product development partner, including in the area of vaccine development, and for its innovative partnerships with the private sector. This made PATH a natural choice to host the Malaria Vaccine Initiative.
Over the years, innovation in vaccine development and introduction has become a leading platform at PATH, one that continues to grow thanks to support from donors such as the Gates Foundation, and UK Foreign, Commonwealth & Development Office. This growth includes the creation of CVIA, which today is working on more than 20 vaccine candidates to protect against more than a dozen pathogens, with many important successes in vaccine development and introduction, including for meningitis A, Japanese encephalitis, and rotavirus.
PATH’s approach
PATH’s involvement in the development, pilot implementation, and broader introduction of RTS,S spans more than 20 years (see figure below). Specifically, PATH has played a role in the following areas:
- Scientific expertise. PATH has extensive experience in vaccine development, expertise in malaria treatment and prevention, and demonstrated success in running clinical trials. Together, PATH and the Malaria Clinical Trials Alliance made critical contributions to the capacity of the research centers conducting the Phase 3 clinical trials to perform high-quality malaria vaccine research. This included building of infrastructure, provision of equipment (e.g., laboratory equipment and X-ray machines), establishment of quality systems, and staff training.
- Project and financial management. PATH partnered with and funded the African research centers that conducted the Phase 3 trial of RTS,S, managing the contracts associated with the trial and, in some cases, working with research centers to strengthen their financial management capabilities.
- Communications expertise. PATH leveraged its health communications capacity to facilitate robust communications planning and ongoing communications support for the clinical trials and dissemination of study results, working with a network of communications officers from the research centers during the trials. PATH communications staff also worked closely with counterparts in the ministries of health during the pilot implementation of RTS,S, and that work is continuing in those countries where PATH is providing technical assistance for vaccine rollout.
- Partnership building. Effective partnerships underpin everything PATH does. The organization has established strong relationships with pharmaceutical companies, leading African research scientists, country ministries of health, nongovernmental organizations, and multilateral organizations like Gavi, UNICEF, and WHO. These relationships were critical to the success of the clinical trials and the pilot program and are critical now to PATH’s work in support of new country introductions, as are its strong links to local partners.
- Market strategy. PATH has been an active partner in efforts to ensure a healthy malaria vaccine market, working with Gavi, WHO, and others, including the effort with GSK to identify a product transfer recipient. Bharat Biotech of India LLC/Ltd. was selected as the recipient in late 2020 and may start supplying RTS,S as soon as 2026; the full product transfer process is expected to be completed by no later than 2029.⁶
- Vaccine program planning and implementation. Even while RTS,S was still in development, PATH teams in Africa helped countries to think ahead about the decision-making framework that would guide national consideration of its introduction. As the vaccine became available, PATH assisted countries in decision-making, identification of the initial areas for introduction, communication and community engagement strategies, and detailed plans for implementation.

The results
RTS,S continues to be administered in Ghana, Kenya, and Malawi following the completion of the pilot program in December 2023 and the transition to Gavi-supported vaccine doses. Across the three pilot countries, more than 6 million doses of RTS,S have been administered since 2019. Additionally, Burkina Faso, Benin, Cameroon, Liberia, and Sierra Leone introduced RTS,S in the first several months of 2024. Other countries plan to introduce RTS,S and R21 in 2024 and 2025.
In addition to the Phase 3 results mentioned above, the RTS,S pilot program demonstrated that use of a malaria vaccine should have a significant positive public health impact. The pilot program documented a 13 percent drop in all causes of mortality among children eligible to receive the vaccine and a 22 percent drop in severe malaria hospitalizations.⁷ In addition, the RTS,S vaccine reached more than two-thirds of children who were not sleeping under an insecticide-treated net, thereby increasing overall access to malaria prevention measures to greater than 90 percent among children participating in the pilot program.
More exciting data also were generated by a Phase 3 clinical trial conducted in areas of Burkina Faso and Mali with highly seasonal malaria transmission. Led by the London School of Hygiene and Tropical Medicine, the study (2017–2022) compared the efficacy of RTS,S to that of seasonal malaria chemoprevention (SMC), the standard treatment for children in areas with highly seasonal malaria transmission. Results after three years of follow-up showed that RTS,S is comparable to SMC in preventing clinical malaria—the latter having demonstrated around 75 percent efficacy—and that combining the two interventions is markedly superior to either intervention alone. Use of the two interventions together resulted in an approximately 70 percent further reduction in malaria deaths and hospitalizations and a 60 percent reduction in uncomplicated malaria over use of SMC alone.⁸
“It was absolutely critical to understand not just how RTS,S performs on its own but how it can best be used in tandem with other interventions for maximum impact. The data from this trial are potentially a game-changer for malaria control efforts,” said Dr. Birkett. “These significant results also helped inform WHO’s recommendation of RTS,S and the testing of other vaccines and monoclonal antibodies.”
In October 2023, WHO added R21 as a recommended malaria vaccine. Prequalified by WHO in December 2023, R21 is expected to move into routine implementation in the latter half of 2024. Its rapid advancement through development and WHO review processes are due in part to the data generated by and the lessons learned through the experience with RTS,S.
Today, the research centers in Burkina Faso, Ghana, Kenya, Malawi, Mozambique, Gabon, and Tanzania that are involved in the Phase 3 trial continue to play key roles in malaria vaccine development and evaluation.
Locations
Benin, Burkina Faso, Cameroon, Gabon, Ghana, Kenya, Liberia, Malawi, Mozambique, Nigeria, Sierra Leone, Tanzania.
Partners
- Bill & Melinda Gates Foundation
- Chevron Corporation
- Clinical Trials Partnership Committee
- European & Developing Countries Clinical Trials Partnership
- ExxonMobil Foundation
- Gavi, the Vaccine Alliance
- German Federal Ministry for Education and Research
- GiveWell
- The Global Fund to Fight AIDS
- Tuberculosis and Malaria
- GSK
- Open Philanthropy
- Unitaid
- UK’s Joint Global Health Trials
- Wellcome Trust
- World Health Organization.
Funding
RTS,S research, development, and implementation have been made possible by a variety of donors throughout the decades-long ongoing process. These donors include the Gates Foundation, Chevron Corporation, European & Developing Countries Clinical Trials Partnership, ExxonMobil Foundation, Gavi, German Federal Ministry for Education and Research, GiveWell, Global Fund, Open Philanthropy, Unitaid, UK’s Joint Global Health Trials, and Wellcome Trust.
References
- Alonso P, Sacarlal J, Aponte JJ, et al. Efficacy of the RTS,S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet. 2004;364(9443):1411–1420. https://doi.org/10.1016/s0140-6736(04)17223-1
- Aponte J, Aide P, Renom M, et al. Safety of the RTS,S/AS02D candidate malaria vaccine in infants living in a highly endemic area of Mozambique: a double blind randomised controlled phase I/IIb trial. Lancet. 2007;370(9598):1543–1551. https://doi.org/10.1016/s0140-6736(07)61542-6
- RTS,S Clinical Trials Partnership. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet. 2015;386 (9988):31–45. https://doi.org/10.1016/s0140-6736(15)60721-8
- Tinto H, Otieno W, Gesase S, et al. Long-term incidence of severe malaria following RTS,S/AS01 vaccination in children and infants in Africa: an open-label 3-year extension study of a phase 3 randomised controlled trial. Lancet Infectious Diseases. 2019;19(8):821–832. https://doi.org/10.1016/s1473-3099(19)30300-7 .
- Dicko A, Greenwood B. Malaria vaccination and rebound malaria. Lancet Infectious Diseases. 2019;19(8):790–791. https://doi.org/10.1016/s1473-3099(19)30282-8
- GSK, PATH, and Bharat Biotech sign product transfer agreement to help ensure long-term supply of RTS,S/AS01E malaria vaccine. Press release. GSK; January 27, 2021. https://www.gsk.com/en-gb/media/press-releases/gsk-path-and-bharat-biotech-sign-product-transfer-agreement-to-help-ensure-long-term-supply-of-rts-sas01e-malaria-vaccine/
- Bawa J, Okine R, Kelleher K. High-impact results from the Malaria Vaccine Implementation Programme (MVIP). ASTMH Annual Meeting 2023 blog. October 20, 2023. Accessed February 2024. https://iamtropmed.org/blog/2023/10/20/malaria-vaccine-implementation-programme-mvip
- Chandramohan D, Zongo I, Sagara I, et al. Seasonal malaria vaccination with or without seasonal malaria chemoprevention. New England Journal of Medicine. 2021;385(11):1005–1017. https://doi.org/10.1056/nejmoa2026330