Products designed for the wide range of women’s health needs—both the innovations marketed for female-specific health conditions and those developed to address conditions that affect women disproportionately or differently—can dramatically improve women’s health and well-being across all life stages. This can include medicines to manage pregnancy-related complications, diagnostics and treatments for women’s cancers, and contraceptive products designed to better meet women’s preferences. When women’s and girls’ health needs are met, they’re more likely to stay in school, join the workforce, and help bolster their economies—all key components to achieving a gender-equal world.
While there have been growing calls for increasing innovations for women’s health care, progress for investing in solutions to improve the health of women and girls, particularly those in low- and middle-income countries (LMICs), hasn’t kept pace with the need. Persistent weaknesses in health systems, including research and development (R&D) driven by the interests of high-income countries, still prevent millions of women from being able to access comprehensive, high-quality, and affordable products and services that fit their preferences.
When LMIC markets are an afterthought rather than the goal, the innovations put forward may not effectively address the health challenges faced by women and girls in low-resource settings. New women’s health products often take years longer to be introduced to LMIC markets than other global health products due to regulatory inefficiencies, resulting in delayed access for women and girls. Gender-based biases in the research stage, such as low representation of women in STEM research leadership positions or insufficient inclusion of pregnant women in clinical trials, also contribute to limited markets and ineffective solutions for addressing the unique health needs of women and girls around the world.
Coordinated advocacy is necessary to address barriers—such as underinvestment, inefficient regulatory systems, and inequitable inclusion and participation—to ensure existing tools reach the women who need them and new tools advance quickly through the R&D cycle to be safely and equitably manufactured, introduced, and delivered.
Developing new tools
PATH has a long history of driving the development and scale-up of user-centered innovations for women’s health. For decades, we have supported the development and delivery of subcutaneous depot medroxyprogesterone acetate (DMPA-SC), a small, easy-to-use injectable contraceptive that gives millions of women autonomy over their own health through the option of self-injection.
Working alongside Sinapi Biomedical, a South African manufacturer, we designed and brought to market the Ellavi uterine balloon tamponade, an affordable, long-term treatment solution to postpartum hemorrhage—a dangerous health issue for which R&D funding has reached new lows in recent years despite being the leading cause of maternal mortality in LMICs.
PATH is also a leader in the self-care movement, driving the development of products and approaches that place people at the center of their own health decisions and ensure women have more user control. In addition to self-injection of DMPA-SC, we have championed the development and delivery of self-testing services as a vital support for women in low-resource settings seeking to gain valuable information about their state of health and pursue next steps according to their preferences.
Through partnerships with the ministries of health and organizations in El Salvador, Guatemala, Honduras, and Nicaragua, PATH accelerated the adoption of human papillomavirus self-testing for cervical cancer, with more than 300,000 women self-screening since 2014. In India, Indonesia, Kenya, Uganda, and Vietnam, we are working with governments to advance HIV self-testing through pharmacies and e-commerce platforms as a way to reach individuals unable or unwilling to utilize facility-based testing services, helping more people understand their HIV status and potentially advancing HIV prevention and treatment in key populations.
While new tools are needed for addressing women’s health concerns, it is important that the technologies are developed in partnership with the users and communities they’re intended to reach. Much of the pipeline for women’s health R&D is driven by the needs and markets of high-income countries, resulting in innovations that cannot always be effectively utilized in low-resource health settings. Without considering the unique needs of women and their communities, even the most well-intentioned innovations will fall short.
For PATH, this means our work for innovative health technologies and approaches is anchored in human-centered design principles. We partner with communities to design, build, and implement health technologies and approaches to ensure the solutions are sustainable, appropriate, and aligned with local priorities. And for women’s health, it means women and girls have a critical role to play in the design of new products intended for them.
Elevating women’s voices in R&D processes
PATH researchers have found again and again how vital it is for women’s voices to be included in the R&D process. While there has been gradual progress, women’s participation and influence in R&D for health remains limited.
“Women and girls have valuable perspectives to offer across all levels of the R&D value chain and need to be represented in the data and institutions that directly impact the safety and efficacy of women’s health treatments.”— Bonnie Keith, Senior Advisor, Reproductive Health
In April 2023, PATH set out to investigate the inclusion of women’s voices in health R&D processes in Kenya and Nigeria. By looking at the enabling laws and policies in place to elevate women’s health R&D, the active involvement of women across all phases of the R&D value chain—from policymakers to researchers and research participants to end-users—and the degree to which women can access capabilities and resources to sustain their leadership and influence in women’s health R&D, our policy experts were able to identify the extent of inclusion of women’s voices in health R&D in these countries, as well as opportunities to resolve existing gaps. Our analysis focused on two primary avenues for amplifying women’s voices within R&D: within the clinical trial data and as leaders in research.
Enabling policies are in place to promote the inclusion of women in health R&D in Kenya and Nigeria, but insufficient implementation of and investment in these policies result in a limitation of the potential impact women and girls can have across the R&D value chain. Up to only 25 percent of active clinical trials in both countries are focused exclusively on women’s health concerns. Cultural norms and practices surrounding women’s roles and responsibilities were identified as a factor limiting women’s participation in clinical trials.
For example, our analysis found that concerns about women’s reproductive health and potential harm to fetuses during trials may result in the exclusion of pregnant women or women of childbearing age from certain studies. This limited inclusion of women in clinical trials means many health products are not designed with evidence available on the safety and efficacy of treatments for women and girls at varying stages of life.
Existing inequities in women’s representation and leadership in health R&D can also be traced back to socio-cultural barriers, often beginning in childhood, such as discouraging or otherwise limiting women’s and girls’ participation in STEM education and careers. Beyond education, opportunities for women to advance in the R&D field through mentorship or accessible research funding are very limited in the two countries, leading to greater disparities in participation and knowledge production.
While more change is needed, there has been promising, if gradual, progress in the establishment of inclusive gender policies and mainstream conversations on gender equalities that highlight the need for greater inclusion of women in science in Kenya and Nigeria. Building on this momentum, advocates in both countries can support efforts to increase the inclusion of women’s voices in health R&D by supporting the development and implementation of gender-inclusive policies, increasing access to and acceptance of STEM education for girls, establishing funded programs to expand opportunities for women pursuing careers in health R&D, and advocating for programs to provide mentorship and networking opportunities.
Increasing access to existing products and services
Creating new health products that reflect the expressed desires of women and girls isn’t enough. Many high-quality, effective services and products exist on the market already, but can be difficult for women to obtain due to barriers such as cost, availability, and accessibility.
A recent PATH study revealed nine market failures impeding access to diagnostics in LMICs, where only 19 percent of patients have access to appropriate diagnostics at the primary health care level, including limited or inconsistent product availability, products of poor or unverified quality, and products that are inappropriately designed or unaffordable. To increase access to existing products and services for women, we must strengthen health systems, including R&D, especially for primary health care services needed to improve women’s health.
Diversifying health product manufacturing so that products are produced closer to the women who will be using them should be explored as an important approach to increase access. With the bulk of women’s health product manufacturers focused on the potential of high-income country markets, it can be very challenging to ensure the R&D pipeline is considerate of the unique needs of LMIC markets. It was a challenge PATH faced in the creation of the uterine balloon tamponade and one of many reasons why we are committed to advancing the new markets and regulatory systems needed to support local production of essential medicines and devices in LMICs.
Changes in the marketplace for medical products and services driven by the self-care movement and the COVID-19 pandemic also present an opportunity to expand access and choice for women’s health. Drug shops, pharmacies, e-commerce, and digital counseling services provide critical access points for women’s health services, but while demand for these services continues to grow, policy has fallen behind. Because some of these approaches are still novel, such as e-commerce sites and digital counseling platforms, very few countries have comprehensive formal policies or guidelines in place for these channels, limiting their ability to scale.
But progress is possible. Through targeted and coordinated advocacy efforts, advocates can work to shape policy agendas and gain policymaker buy-in. As these health service channels are not limited to servicing only women’s health needs, advocates for women’s health innovations should align wherever possible with the broader movements for strengthening health systems and primary health care. With an integrated approach to updating guidelines and policies, especially for emerging services like digital health and telemedicine, access to existing products and services can be expanded via these channels.
Accelerating change through advocacy
Advocacy driven by cross-sector partnerships is the key to influencing policies that prioritize and incentivize innovations that improve the health of women; mobilizing resources and support to ensure the inclusion of women’s voices in health R&D at all stages; and improving access, availability, and affordability of existing products.
Leveraging the findings from our exploratory research, PATH developed a framework to support coordinated policy advocacy for women’s health R&D innovation.
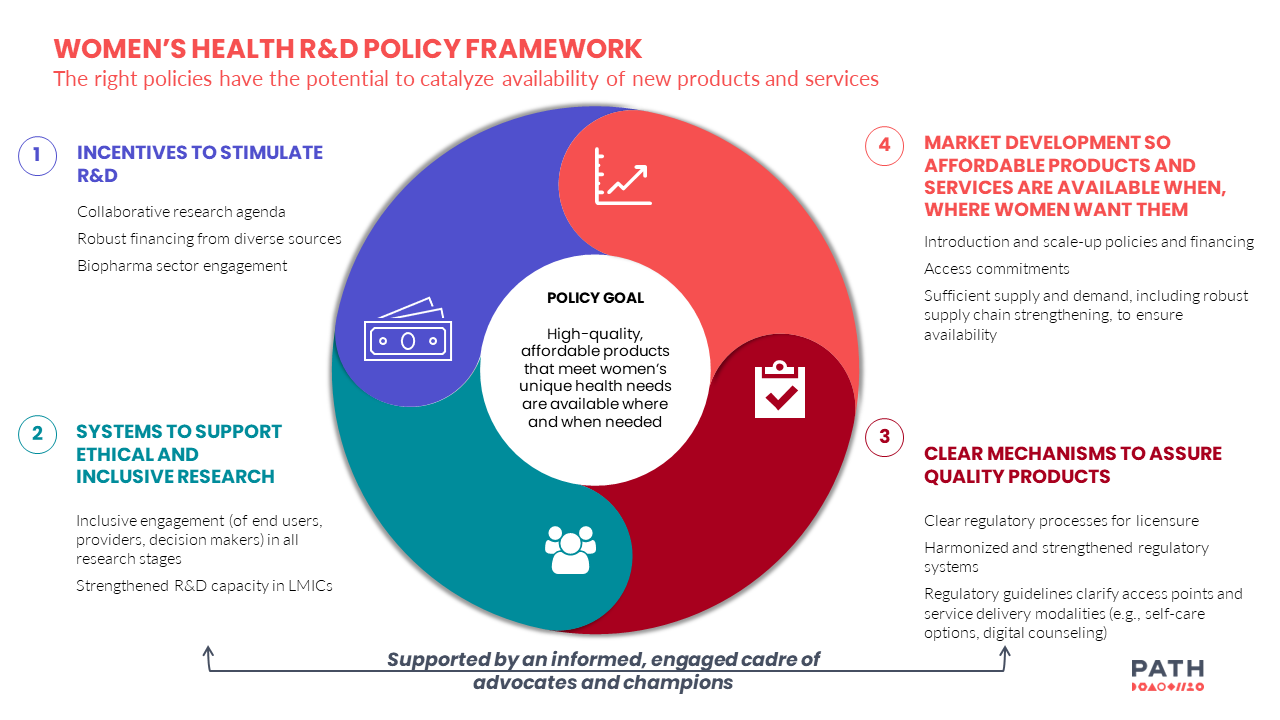
The right policies—including incentives to stimulate women’s health R&D, systems to support ethical research that integrates the views and priorities of women and girls, clear regulatory processes, and market development—have the potential to expand women’s health innovations when coupled with scientific progress, so women and girls around the world can have access to the products that support their unique health needs according to their preferences.
We’ll continue to advocate for parallel investments in new tools and systems strengthening to improve the health of women around the world. PATH applauds the recent Women’s Health Innovation Opportunity Map report by the Innovation Equity Forum, which highlights 50 high-return opportunities to advance global women’s health R&D and bring women’s health to the forefront.
For more information on how PATH leverages advocacy and public policy to transform health systems and resources for advocates, visit our Advocacy Resource Hub or contact us at advocacyandpolicy@path.org.



