Improving detection and accelerating elimination.
When caring for someone who is sick, the first step is often running a diagnostic test. In low-resource settings, diagnostic testing is often completed using small, easy-to-operate rapid diagnostic tests (RDTs), many of which require only a finger prick of blood and provide results in a matter of minutes. However, RDTs have sometimes had a lower sensitivity than laboratory-based testing methods. This means some RDTs will not detect cases that a laboratory test can detect. PATH is working with partners across the globe to increase the sensitivity of RDTs so that fewer cases are missed. More sensitive diagnostic tests are also key components of working toward disease elimination—or even eradication.
Take malaria, a disease the World Health Organization (WHO) estimates caused 435,000 deaths in 2017. Malaria RDTs are important to case management and elimination, but they historically have had lower sensitivity than some laboratory-based malaria tests. PATH has collaborated with researchers and diagnostics developers to improve the sensitivity of malaria RDTs, such as a test launched in 2017 that is ten times more sensitive than previous tests. And PATH continues to collaborate with partners to develop more sensitive malaria RDTs.
Beyond developing tests to detect malaria, PATH is supporting diagnostics that help guide appropriate treatment for malaria in order to both improve health outcomes and break the cycle of transmission. By advancing the development of point-of-care tests for glucose-6-phosphate dehydrogenase (G6PD) deficiency—a genetic disorder affecting approximately 400 million people—PATH is making it possible to determine if an important treatment for Plasmodium vivax malaria is appropriate for a patient. The treatment, which uses 8-aminoquiline drugs to completely kill all forms of the P. vivax malaria parasite, prevents malaria relapses that can occur weeks or months after the initial infection and ongoing infectivity. However, people with G6PD enzyme deficiency are at risk for severe negative reaction to the medication, so testing before treatment is required. Along with more sensitive RDTs, G6PD testing will be an important tool as countries push to eliminate P. vivax malaria.
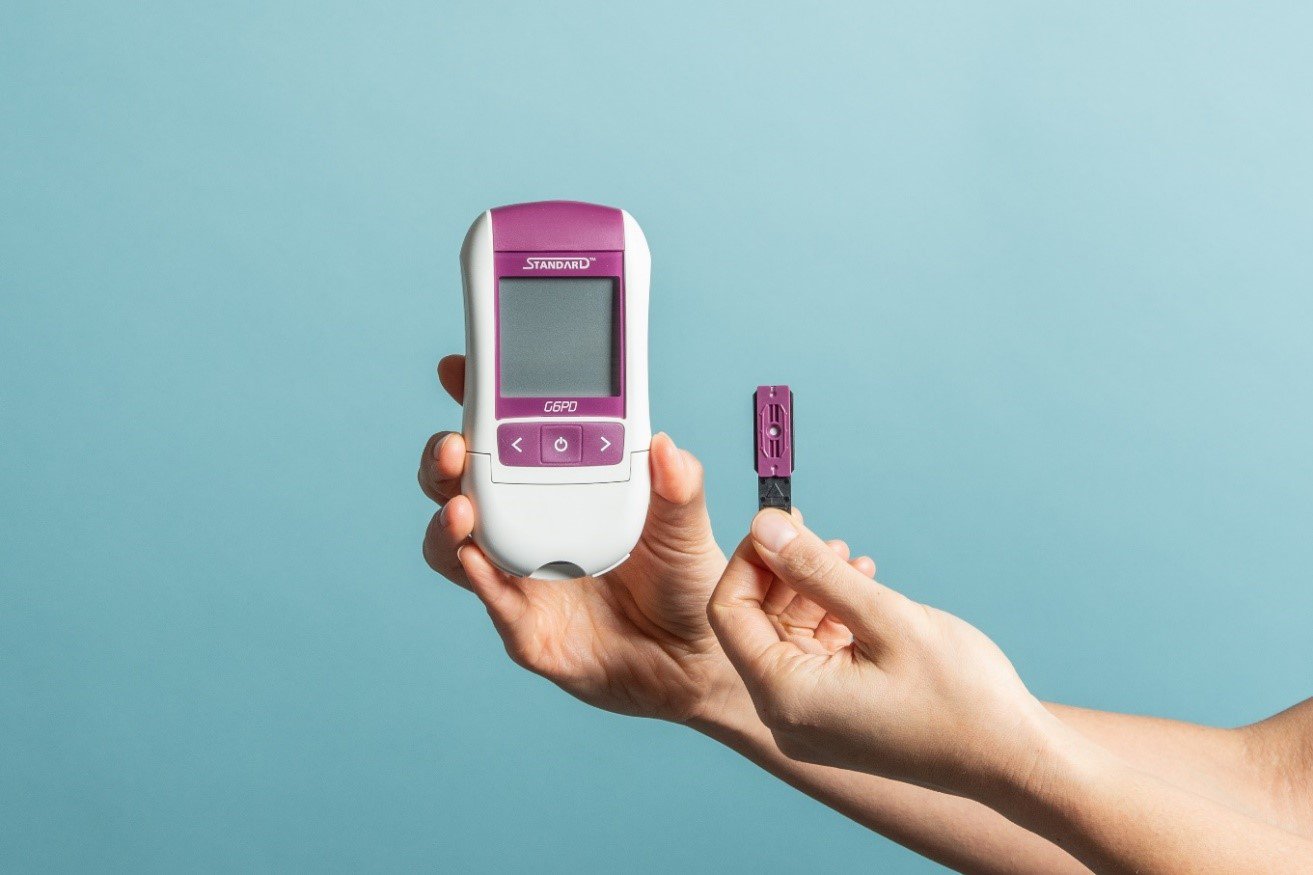
PATH has supported the development and commercialization of a portfolio of G6PD diagnostics, including SD BIOSENSOR’s STANDARD™ G6PD Test. Photo: PATH/Patrick McKern.
Strengthening epidemic preparedness.
From Ebola to Zika, yellow fever, and cholera, emerging and re-emerging diseases have the power to grow into public health crises. Diagnostics are critical to preventing the spread of outbreaks. When grounded in a strong health system as part of a robust surveillance program, diagnostics help detect emerging threats early so that a response can be mobilized.
The challenge is that appropriate diagnostics are often unavailable for diseases that pose an outbreak risk. By developing appropriate diagnostic tools for high-priority pathogens and strengthening laboratory capabilities before the next outbreak, epidemics can be more effectively controlled, particularly in low-resource settings. PATH partners with researchers to ensure the right diagnostic tools are created to prepare the world for epidemics.
For example, PATH worked with the University of Washington and other partners to develop an innovative way to detect polio in the environment in challenging settings, such as remote and mountainous regions. The role of epidemic preparedness, including effective diagnostics, is underscored in a globally connected world where pathogens can spread quickly.

Diagnostics play an important role in surveillance and epidemic preparedness. A lab worker runs a test in a regional hospital in Thies, Senegal. Photo: PATH/Gabe Bienczycki.
Providing user-friendly testing options.
Diagnostic tests are performed by a wide range of users—from scientists in laboratories to frontline health workers using RDTs. A key challenge is making sure that diagnostic tools can be used effectively by all users—from performing a test to interpreting the results. Developing diagnostic tools that are focused on the needs of the users is at the core of PATH’s approach.
Designing for end users is particularly critical for self-testing. Self-testing has long been a part of health care—think of a thermometer identifying a fever. It is now also being used as a strategy to provide access to diagnostic testing for groups that have a high risk, or may not seek testing through a health care provider due to stigma, geography, or many other reasons. For example, PATH has collaborated with partners to bring user-focused community HIV testing (lay and self-testing) to Vietnam.

A focus on end users is central to PATH’s work in diagnostics. This is all the more important when developing self-testing options like the HIV test shown above. Photo: PATH/Ian Taylor.
Connecting diagnostics digitally for greater impact.
Digital health tools—from electronic health records to user apps—can be powerful solutions that help improve health outcomes, increasing the effectiveness and efficiency of health systems and ensuring individuals get the best care. “Connected diagnostics” link the vital role of diagnostics in health with the power of digital health, often by augmenting the role of a diagnostic tool with additional digitally enabled functionality.
For example, a diagnostic test that traditionally produces an analog result can be adapted so that it produces a digital result, which can rapidly be combined with nationwide data to guide public health resources and actions. A second important use for connected diagnostics that PATH is actively exploring is decision support for health care workers—a digitally enabled way to guide treatment decisions based on the results of a diagnostic test. These applications are only the tip of the iceberg for connected diagnostics. Future use could include analytics and machine learning to help select the best intervention or to predict future disease outbreaks.
These four innovations only begin to tell the important story of how diagnostics help us improve health outcomes and health equity. In fact, WHO states that diagnostics “are essential for advancing universal health coverage, addressing health emergencies and promoting healthier populations.” Recognizing the importance of diagnostics, WHO created the Model List of Essential In Vitro Diagnostics. While the concept of essential medicines is familiar to many people (WHO created its first list of essential medicines in 1977), the essential diagnostics list is relatively new—the first edition was published in 2018. As countries work to expand access to the lifesaving diagnostics on the list, PATH is excited to continue its efforts to improve current diagnostic tools and develop the next generation of technologies.
Learn more about PATH’s work in diagnostics.



