Using human-centered design to develop a female condom that meets users’ needs
Women experience significant morbidity and mortality in many countries due to unintended pregnancy, HIV, and sexually transmitted infections. Although male condoms can provide this protection, gender inequality and cultural issues make it difficult for women to negotiate their use.
Problem Definition
Design & Development
Prototype Evaluation
Product Validation
Market Research & Commercialization

The challenge
Women experience significant morbidity and mortality in many countries due to unintended pregnancy, HIV, and sexually transmitted infections (STIs). Currently, condoms are the only contraceptive method that also protects against HIV and STIs. Still, gender inequality and cultural issues often make it difficult for women to negotiate their use. Female condoms, however, offer a woman-initiated option that protects from pregnancy as well as HIV and STIs. A female condom that offers good sensation and is acceptable and attractive to both partners has the potential to reduce significant barriers experienced by women when negotiating condom use for safer sex.
The approach
Using a human-centered approach that incorporated users as co-designers throughout product development and testing, PATH developed an innovative female condom with features inspired by user input to address a desire for a product that provides protection and is simultaneously acceptable, easy to use, and pleasurable for both partners. This approach would also ensure that couples would be more likely to use the product correctly and consistently, thereby increasing their overall protection against sexually transmitted diseases, including HIV and unintended pregnancy.
Understanding the motivations, ideas, and constraints of women and their partners in culturally representative sites in Thailand, Mexico, South Africa, and the United States allowed PATH to partner with end users to co-develop an approach to design and develop a female condom that was genuinely appropriate and reflective of local priorities.
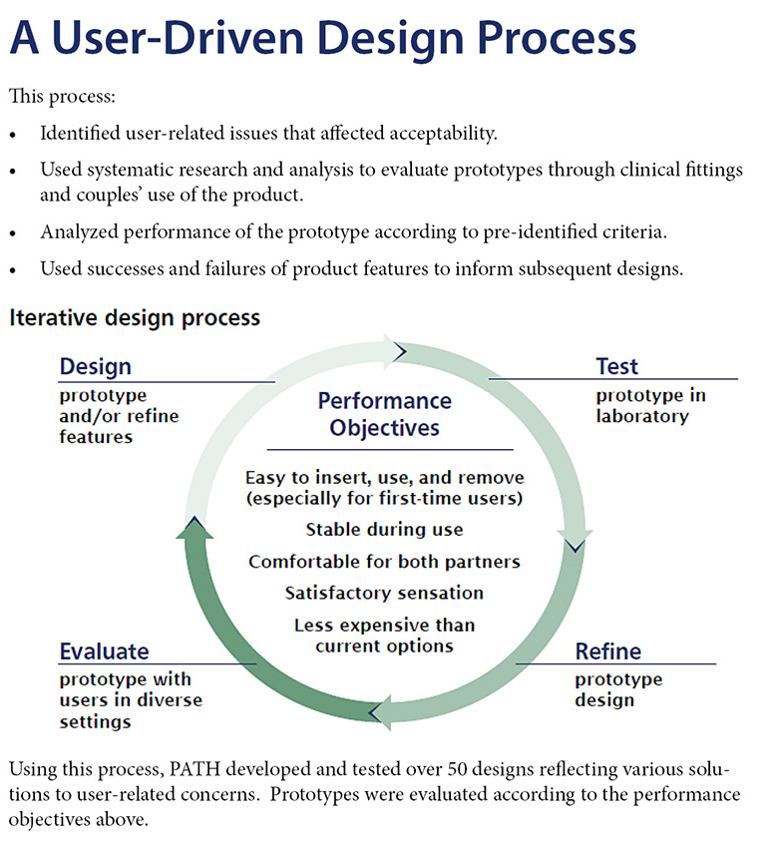
Through a human-centered design approach, a multidisciplinary team embarked on a participatory design process that included a desk review of currently available female condom products and formative research with women’s groups, health care providers, and sexual and reproductive health advocates to identify the performance objectives required to meet consumer needs best. These objectives provided a road map for developing a product that addressed challenges with existing products and set the benchmark against which users in an iterative process evaluated prototype designs. Product requirement specifications were developed to define evaluation indicators for functional performance and acceptability.
This iterative process started with testing materials and product features to understand the user requirements regarding better product sensation, ease of handling, and functionality that users wanted from an improved female condom design. Features were tested through iterative rounds of evaluation to inform development. The team systematically collected user-testing data during observations at clinical fittings where women and providers evaluated ease of insertion, fit, comfort, removal, and acceptability. Features that best met users’ needs were combined into prototypes evaluated by couples’ use during sex.
After each round of prototype evaluation, the team collected feedback from women and their partners via open-ended questionnaires and in-depth interviews. Evaluations included both couples from the previous round of evaluation as well as couples with no previous experience. This helped assure that refinements in new prototypes actually met the couples’ needs from the previous evaluation as well as building a fresh user group for additional insights. We completed multiple rounds of iterative evaluation in all countries to inform the Woman’s Condom design until all product specifications were met.
Across clinical fittings and couples’ use, over 50 design iterations of the Woman’s Condom were tested among more than 200 women from four countries. This allowed designers to iteratively address solutions to user requirements for ease of insertion, comfort, sensation, and condom stability during use. Studies in multiple countries subsequently confirmed the design was safe and was well accepted by both partners, and in some cases preferred over other female condoms. Clinical studies also confirmed that Woman’s Condom performs as well as other female condoms in terms of barrier protection.
PATH’s design process assessed product materials and manufacturing during design development to ensure that designs moved forward would be manufacturable and could reach a unit cost that would meet market requirements when produced at scale. Since the female condom market consists primarily of international procurement by development agencies and governments for use in family planning and HIV programs in low- and middle-income countries, cost constraints for the product were a critical consideration.
The solution
The Woman’s Condom is a thin plastic pouch inserted in the vagina before intercourse and stays in the vagina during intercourse, providing a physical barrier between partners' genitalia. It is unlubricated and designed for single use.

In 2008, PATH licensed the Dahua Medical Apparatus Company (Dahua) of Shanghai, China, to manufacture and distribute the Woman’s Condom. From 2010 to 2015, PATH worked with Dahua on manufacturing scale-up, regulatory applications, and preparation for market introduction in China and South Africa, with funding from the UK Department for International Development (now the Foreign, Commonwealth & Development Office). During the same time, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) implemented a contraceptive effectiveness study of Woman’s Condom at ten study sites in the United States, generating clinical data required for a regulatory application to the US Food and Drug Administration.
In 2016, Dahua’s Woman’s Condom achieved prequalification from the United Nations Population Fund/World Health Organization Prequalification Programme for Female Condoms. This internationally recognized certification ensured that the Female Condom was of sufficient quality to be procured by international agencies such as the United Nations, often a tender requirement of country governments. Unfortunately, the Woman’s Condom was not successful at competing for the international public sector tenders.
Although we designed Woman’s Condom to be cost competitive at full-scale production, other female condom products are less expensive than Woman’s Condom—in part due to Dahua’s lower initial production volume but also due to Woman’s Condom materials and manufacturing process. Although pilot introduction of Woman’s Condom by the Expanding Effective Contraceptive Options project in Malawi and Zambia (2015–2018) showed good uptake and acceptability, Woman’s Condom currently is stalled in terms of marketing and not available internationally. If results from the NICHD contraceptive effectiveness study are strong, this could potentially attract interest from a company that sees value in developing markets for Woman’s Condom among consumers who have income to pay for pleasurable protection, similar to how companies have developed markets for pleasurable male condoms.
“I like the comfort, stability, and the way it looks.”— Female condom user, South Africa
The impact of using human-centered design
Incorporating diverse user perspectives into the product development process built acceptability into the Woman’s Condom at each step of the process. Allowing a wide range of user needs and perspectives to drive product development helped to ensure that the final product was easy to use, comfortable, and acceptable to both partners. The unique design features of the Woman’s Condom enable easy insertion, secure fit during use, good sensation, and easy removal, resulting in many couples preferring this product to other female condom designs.
At this point, Woman’s Condom is a story of a well-designed product that meets user needs but did not meet the cost-competitive environment for international tenders. Given the strong consumer acceptability of Woman’s Condom, we hope to identify a partner who is willing to develop the market for a middle-priced female condom and market to consumers able to pay for pleasurable protection. Dahua also is exploring a lower-cost version of the Woman’s Condom.
Our partners
- Department of Community Nursing, Khon Kaen University, Khon Kaen, Thailand
- National Institute of Public Health of Mexico, Cuernavaca, Mexico
- MatCH Research Unit, Durban, South Africa
- CONRAD
- Eastern Virginia Medical School, Norfolk, Virginia;
- Harborview Medical Center, University of Washington, Seattle, Washington