Editor's note: A version of this article was originally published on April 5. It has been updated to include information about the Delta and Omicron variants.
The vaccine implications of new SARS-CoV-2 variants have dominated recent headlines, but one team at PATH is focused on a different set of implications: those for tests used to detect the coronavirus.
“With [current] COVID-19 diagnostics, we were on a time crunch, we had to get something out there,” PATH Scientific Officer Lorraine Lillis, PhD recently told The New York Times. “Normally, diagnostics take a long, long time, and we’d normally challenge them with multiple variants. We’re doing that, but we’re doing it in real time.”
We spoke with Lorraine and other scientists from PATH’s Diagnostic program to answer key questions about the Delta variant, the implications for diagnostic tests, and the future of disease surveillance.
“Like all viruses, the coronavirus takes control of cells in its host—infected human beings—and it uses those cells to print more copies of itself,” says Troy Leader, PhD, senior scientific officer in PATH’s Diagnostics program.
“Each new copy of a virus contains tiny copying errors or variations in its genome—hence, variants. Most of these variations are insignificant, but occasionally, one offers a significant evolutionary advantage. It might be harder for immune systems to detect. It might be more transmissible. And quite rapidly, it can become the dominant strain.
“Just look at the explosion of cases in Lusaka earlier this year—that’s a clear example of a new, more transmissible variant taking hold, and that’s why the world has been paying close attention to the genomic surveillance PATH is doing with the University of Zambia.”
Q: What do we know about the Omicron variant?
The new coronavirus variant B.1.1.529 was first identified in South Africa and has been labeled the "Omicron variant" by the World Health Organization.
While it will likely be weeks until we know about the variants’ reinfection potential, antibody resistance and susceptibility to vaccines, the WHO did share that, this variant has 30 mutations to the spike protein, the part of the virus that binds to cells in the body which could have implications for vaccine efficacy and transmissibility. In a recent statement, the organization also shared that it has also been detected at faster rates than previous surges in infection, suggesting that this variant may have also a growth advantage.
According to the WHO, the widely used PCR tests continue to detect infection, including infection with Omicron. Studies are ongoing to determine whether there is any impact on other types of tests, including rapid antigen detection tests.
"What's really promising is that, in comparison to previous variants of concern, this time we're seeing manufacturers of PCR and antigen tests provide more rapid statements about the compatibility of their tests with Omicron," says Neha Agarwal, associate director of PATH's Diagnostic program. "This type of real-time data and awareness is really helpful to health care providers and consumers."
Q: What about the Delta variant? Why are experts so worried about it?
According to WHO, the Delta variant (B.167.2) is the “fastest and fittest” variant yet—as much as 50 to 60 percent more transmissible than the Alpha variant (B.1.1.7), which was already 50 per cent more transmissible than the original strain of COVID-19.
“The Delta variant poses a serious risk to people who are not fully vaccinated, as it is highly contagious.” Lorraine says. “There is also some indication that the Delta variant may result in more severe disease.”
A study in Scotland, published in The Lancet, found the hospitalization rate of patients with the Delta variant was about 85 percent higher than that of people with the Alpha variant.
That’s a troubling statistic for all countries around the world, but particularly for those with limited medical infrastructure. Right now, cases of the Delta variant have led to a surge of deaths on the African continent—most notably in South Africa where less than 23 percent of the population is fully vaccinated. These low vaccination rates are also believed to be a contributing factor to the emergence of the Omicron variant.
Q: With the variants of concern, will current COVID-19 tests still work?
“As you’d expect, the answer depends on the test and the variant,” says Lorraine Lillis, PhD, scientific program officer with PATH’s Diagnostic program.
“For the variants you’ve heard about in the news so far—Alpha, Beta, Gamma, and Delta—most antigen-based tests will continue to work, because most are targeting the N antigen of the virus. And so far, the N antigen remains conserved in these variants. Verifying performance with the newly described Omicron variant is still in the early stages, however no significant issues with antigen tests have been reported to date."
“For molecular tests, whether or not they continue working will depend on the number and location of genes that the test targets. The new variants, including Omicron, have mutations in the S gene that have been found to impact some assays targeting this particular gene. However, if a test is targeting one or more conserved regions other than the S gene, then the test can be presumed to detect these new variants.
“For tests that only target the S gene, we just don’t fully know yet. Additional studies are required.”
Q: This all sounds complicated. How can health officials keep track of what works and what doesn’t?
“It is very complicated,” says Olivia Halas, program coordinator, “which is why the PATH team has expanded our COVID-19 Diagnostics Dashboards to shed light on what we know and don’t know about the variants and their impact on testing.
“Our new variant-specific dashboards—one for molecular tests and one for antigen tests—track key variables such as assay multiplicity and gene or protein target sites. And they help users understand which tests are presumed or confirmed to work with three of the most common variants (Alpha, Beta, Gamma, Delta, and Omicron)," says Olivia. "The dashboard is continuously evolving as more information on diagnostics and variants becomes available, with information on what we currently know on performance of the diagnostics with Omicron now being added.”
Lorraine adds, “Of roughly 56 molecular and antigen tests we’ve assessed, 46 had publicly available manufacturer information on the effectiveness of detecting the Beta variant (initially reported in South Africa), and 41 on the Gamma and Delta variants (initially reported in Brazil/Japan and India, respectively)."
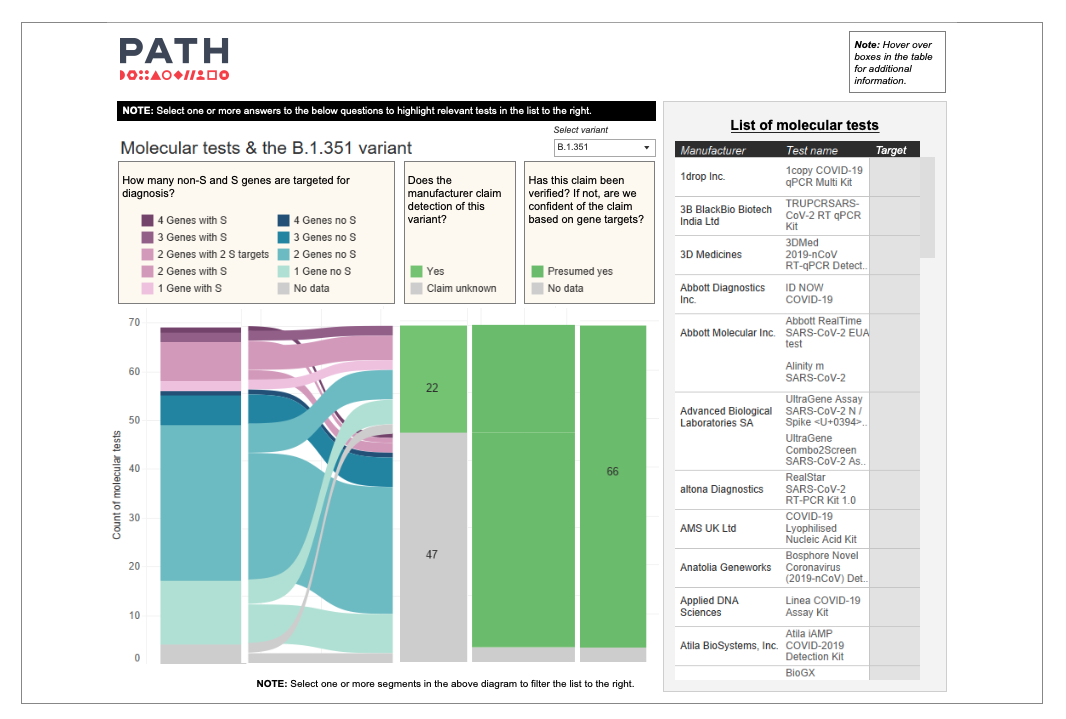
A static image of PATH's new variant-specific molecular diagnostic dashboard. To see the live dashboard, click the text links above. Photo: PATH.
“Everyone’s needs are different,” Neha says, “so our dashboard enables each health official to decide for themselves which tests are best suited for their needs, without having to comb through individual product inserts, manufacturer websites, or research studies. We’ve aggregated all the publicly available information in one place.
“So far, the response to the dashboard has been overwhelmingly positive. If we can secure additional support, we hope to continue monitoring mutations and their implications on diagnostic testing. Right now, we're currently expanding dashboard data to include diagnostics capable of detecting the Omicron variant.”
Q: If more variants are bound to emerge, how can the world get ahead of the pandemic?
“Basically, we need more of everything,” says Troy. “First and foremost, the world needs more COVID-19 testing in more places, and if possible, with faster results—particularly in countries where diagnostic testing remains limited.
“As Neha and Lorraine explained, the dashboard plays a critical role in helping public officials make informed decisions when procuring tests for their health systems. But upstream of that, an incredible amount of work and global collaboration goes into creating appropriate, quality-assured diagnostics in the first place.
“At PATH, we accelerate the creation of new diagnostics by de-risking development wherever we can. In the case of COVID-19, we’ve created a biorepository of more than 20,000 qualified clinical samples that test developers can use to validate their tests. We’ve also established a standardized benchmarking panel for evaluating antigen test performance.
“But these sorts of efforts need to go far beyond COVID-19 and the work we’re doing at PATH. The world needs more robust disease surveillance systems all-up—including greater capacity for genomic sequencing in more places. It’s not an accident that new SARS-CoV-2 variants were first spotted in places that have the genomic sequencing capacity to search for them.
“In this pandemic and the next, stronger disease surveillance systems will be essential—not only for detecting new variants as they emerge but also to monitor the impact those new variants have on the effectiveness of our efforts against them: whether distancing measures, vaccines, or anything else.”
Q: What is PATH doing to strengthen disease surveillance systems?
“Beyond our work with the dashboard and with diagnostic development, our team is exploring innovative approaches to rapidly expand disease surveillance data on variants and other pathogens in settings with limited clinical testing,” says Troy.
“For example, we’re working on new environmental surveillance approaches that enable characterizing variants within sewage or other environmental samples. This is a fast, accurate, and affordable way to take a snapshot of what is circulating in a community rather than characterizing viruses collected from individual patients.
“We’re also working to strengthen other critical components needed for effective disease surveillance—including working with national and international partners through efforts in our Global Health Security Initiative that support the strengthening of other key health system components, such as the data reporting systems required to collect and leverage disease surveillance information in public health decision-making.”
Learn more about PATH’s work in diagnostics.



