For a disease with no treatment, prevention is key
Transmitted to humans by mosquitoes, Japanese encephalitis (JE) begins like the flu and may progress to a brain infection, when it will kill up to 30 percent of its victims and leave thousands more with permanent brain injuries.
There is no treatment. The only solution is prevention through immunization. But until PATH launched our work in JE in 2003, tens of thousands of children died or were disabled each year for lack of an accessible, affordable, and reliable vaccine.
“3 billion people live in areas at risk for JE, and up to 68,000 clinical cases are reported annually.”
Because the disease mainly strikes poor rural communities throughout Asia and the Western Pacific, it historically received little attention. Yet approximately 3 billion people live in areas at risk for JE, and up to 68,000 clinical cases are reported annually.
Up to half of survivors suffer permanent neurological damage, such as paralysis, recurrent seizures, or the inability to speak.

Using data to bring understanding—and pave the way for a solution
In the early 2000s, PATH began suspecting that JE was a bigger problem than previously realized. Health workers in India routinely voiced concerns about JE, and the country’s improved disease surveillance suggested a high incidence.
In 2003, we secured a grant from the Bill & Melinda Gates Foundation to tackle it. Harnessing our expertise in accelerating access to lifesaving vaccines, our goal was to understand the disease, determine the health and economic burden it placed on countries, and prepare the way for a vaccine that would safely and affordably prevent it.
PATH began by helping private-sector partners develop standard diagnostic tests. We worked with the World Health Organization (WHO) and governments to set up surveillance systems and a web-based platform for sharing data about JE incidence. These efforts allowed countries to understand the extent of the disease, prioritize it, and focus prevention efforts on the regions and people most needing protection.

Women transporting Japanese encephalitis vaccines in India. Photo: PATH/Julie Jacobson.
Vaccines against the disease already existed. Some countries had JE vaccination programs in place, and international travelers to Asia were routinely vaccinated against the disease. But the most commonly used vaccine had significant drawbacks—multiple doses were required and it was expensive to produce. There simply wasn’t enough funding or doses available for all the children who needed it.

In search of a solution, PATH surveyed the field for a better JE vaccine. We discovered that China had already developed an affordable vaccine (now known as CD-JEV) and had protected more than 200 million children through immunization. The vaccine, made from active but weakened virus, was safe, effective, and required only one dose. But it was little known outside the country—and might have stayed that way had PATH not figured out how to bring it to other countries of high need, while also playing a bridging role between partners around the world.

CD-JEV is unpacked in India for vaccination against Japanese encephalitis. Photo: PATH/Julie Jacobson.

A deadly outbreak brings new urgency
Then, in 2005, a devastating JE outbreak killed nearly 2,000 people in India and Nepal, mostly children. Media coverage focused international attention on JE, while governments strengthened their resolve to protect their young people. PATH and our partners were able to use this newfound attention around the disease to advocate for introduction of CD-JEV in affected countries.

Preparing the vaccine for rollout
With the vaccine identified, PATH collaborated with international partners and ministries of health in Asia to accelerate its introduction. Because CD-JEV had not been used widely outside of China, international officials called for specific clinical studies. PATH collaborated with the manufacturer, WHO, and ministries of health on pivotal clinical trials to help prove the vaccine was safe and effective.
We also supported clinical trials to confirm that the vaccine can be given at the same time infants get their measles shots, making it easier to fit into existing immunization programs. To help countries plan for the introduction of CD-JEV, we modeled the cost-effectiveness of immunization strategies.
“With the vaccine identified, PATH collaborated with international partners and ministries of health in Asia to accelerate its introduction.”
The vaccine also needed to be affordable for use in low-income countries, so PATH negotiated with the vaccine manufacturer—Chengdu Institute of Biological Products (CDIBP)—to establish a special public-sector price.


Students from Basic Education Middle school No. 13 line up to receive Japanese encephalitis vaccines in Shan State, Myanmar. Photo: PATH/Thet Htoo.
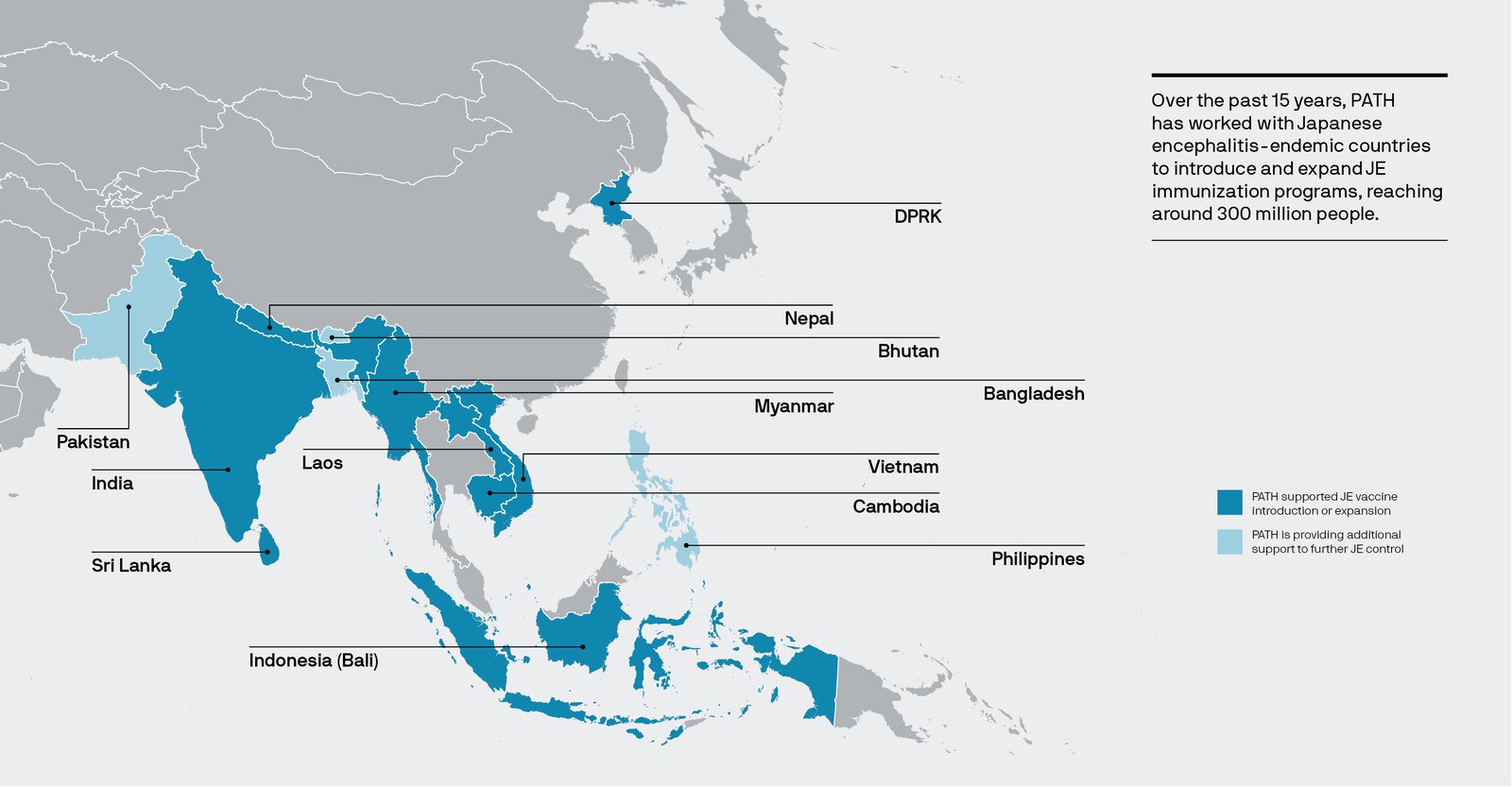
From Cambodia to India: 300 million children are vaccinated
PATH has helped support JE vaccine introduction or expansion in ten countries outside China. India and Nepal were among the first countries to introduce CD-JEV through mass vaccination campaigns and routine immunization services. They were later followed by others, including Cambodia, Indonesia, Laos, and Myanmar.
Along the way, PATH and our partners were there to support the countries—from developing introduction strategies to implementing and evaluating immunization programs.
To date, CD-JEV has reached more than 300 million people outside of China.

Helping China become a player in global vaccine production
We were also helping the vaccine manufacturer, CDIBP, pursue WHO prequalification of CD-JEV as the critical next step in expanding access. Both the vaccine and CDIBP underwent rigorous inspections to ensure they met international standards for quality, safety, and efficacy. PATH provided CDIBP with technical and financial support to meet these standards. We also assisted in the design and financing of a new, high-quality CD-JEV manufacturing facility to ensure production of an adequate amount of this affordable vaccine.

WHO gave its critical stamp of approval in 2013. Prequalifying the vaccine allows United Nations procurement agencies to purchase the vaccine. It also opened the door for the vaccine’s inclusion in the portfolio of Gavi, the Vaccine Alliance, which provides vaccine financing for low-income countries where the burden of JE is especially high.

Medical equipment and vaccines are prepared for Japanese encephalitis vaccination campaigns. Photo: PATH/Aaron Joel Santos.

In 2015, Laos became the first country to receive Gavi support to complete a nationwide JE vaccination campaign, building on earlier campaigns supported by PATH and our partners that reached children in eight provinces. Following the campaign, the government included the vaccine in its routine immunization program.
The JE vaccine also marks China’s entrance into the global vaccine marketplace. CD-JEV is the first vaccine ever produced in China to receive WHO prequalification, with the potential to foster a more competitive vaccine manufacturing market and help shift how vaccines are made, delivered, and priced for the developing world. And because CDIBP and its parent company now have a global export market for their vaccine, they’re advancing China’s role in global health and development.

PATH provided technical assistance to Myanmar’s central EPI for the country’s first immunization campaign against JE and subsequent integration of JE vaccine into the national routine immunization program. The campaign, conducted in two phases, reached more than 12 million children, or 92.5% of the country.


Health care workers are a vital part of ensuring children in rural and hard-to-reach communities receive JE vaccine. Photo: PATH/Aaron Joel Santos
PATH, in collaboration with the Laos Ministry of Health, launches “Sustaining the Japanese Encephalitis Vaccination program” project to strengthen the country’s immunization system and undertake a multi-year effort to reach children who previously went unvaccinated in three provinces.
By the end of the project, three years later, nearly 190,000 children in the three provinces had been vaccinated against JE.

By 2020, 15 countries had national or subnational public health vaccination programs in JE endemic areas.

The Government of Laos grappled with the impact of the COVID-19 pandemic, and PATH drew upon existing JE project resources, as well as local networks and connections, to strengthen the immunization system and prepare for the rollout of COVID-19 vaccines. PATH quickly supported the government to ensure health care workers and local communities were ready for COVID-19 vaccine rollout. In direct partnership with the Government of Laos Ministry of Health, Department of Planning & Finance, University of Health Science, and support from a local telecon provider, PATH built an e-learning platform in local languages, with videos and subtitles, to bolster training for health workers about routine immunization, including JE vaccine, COVID-19 vaccination, as well as stock management and communications.
Once tested and fully built, the e-learning platform, with four complete modules, rolled out at a central level, followed by cascade trainings within each province. By November 2022, all 18 provinces had received their training. Health workers have 24/7 access to the platform to receive information, access support and connect with centralized information.
In late 2022, PATH fully handed over the e-learning platform to the Government of Laos. The telecon system is fully supported locally and is under the control of the National Immunization Program. PATH's technology support team in Hanoi, Vietnam provided technical assistance as needed. The Government of Laos expanded the e-learning beyond COVID-19 and JE vaccines so that health workers have access to routine immunization information when they need it.

A family with their vaccination cards at the Japanese encephalitis vaccination campaign in Khon Kahndone Village, Xieng Khouang Province, Laos. Photo: PATH/Aaron Joel Santos.
1 of 4
Families fill out paperwork as they arrive to be vaccinated against Japanese encephalitis. Photo: PATH/Aaron Joel Santos.
2 of 4
Orladee Nonnavongsa gets ready to work at the Japanese encephalitis vaccination campaign. Photo: PATH/Aaron Joel Santos.
3 of 4
CD-JEV offers critical protection from Japanese encephalitis for children. Photo: PATH/Aaron Joel Santos.
4 of 4Equity for the world’s children through vaccines
At PATH, we believe access to vaccines is critical to achieving health equity. And we’re committed to ensuring countries have the tools they need to thrive. Our Center for Vaccine Innovation and Access (CVIA) has expertise at every stage of the complex process of vaccine research, development, and introduction. This allows us to both accelerate access to existing vaccines and make new vaccines rapidly and widely available.
“Our Center for Vaccine Innovation and Access (CVIA) brings together diverse experience and expertise in both disease and functional areas.”
The story of PATH’s JE work collaborating across sectors to bring a critically needed vaccine to a global marketplace is a prime example of our unique, comprehensive approach. Currently, we work across more than two dozen vaccines in development or already in use, spanning 17 different diseases.



