Maternal and infant mortality are key indicators of a community’s health. When health care providers are equipped to quickly respond to childbirth complications and monitor newborn vital signs, some of death’s leading causes become preventable.
That’s why India’s government is seeking new, high-impact devices and tools that can reach the hands every health care provider in the country. PATH and our partners are supporting these efforts with our extensive expertise in maternal and child health, and more than 40 years of experience developing and introducing critical health products and facilitating their distribution and adoption.
Though each country, city, and community on Earth has its own unique health challenges, a few common principles remain the same whether you’re introducing and scaling up health innovations in New Delhi, Nairobi, or Yangon.
1. Make options affordable
When novel, lifesaving, health devices don’t reach the care providers that need them, it’s often a result of market failures. When lifesaving products are unaffordable, they are inaccessible. And accessibility is a central barrier to improving health.
PATH works with governments, scientists, engineers, manufacturers, and other partners at every stage of the development process to ensure cost-effective options that increase the accessibility of new health technologies.
For example, the uterine balloon tamponade (UBT) is an important tool for managing postpartum hemorrhage, but many designs are prohibitively expensive. PATH and South African manufacturer Sinapi Biomedical co-developed the Ellavi UBT with assistance from physicians, midwives, and other maternal health experts.
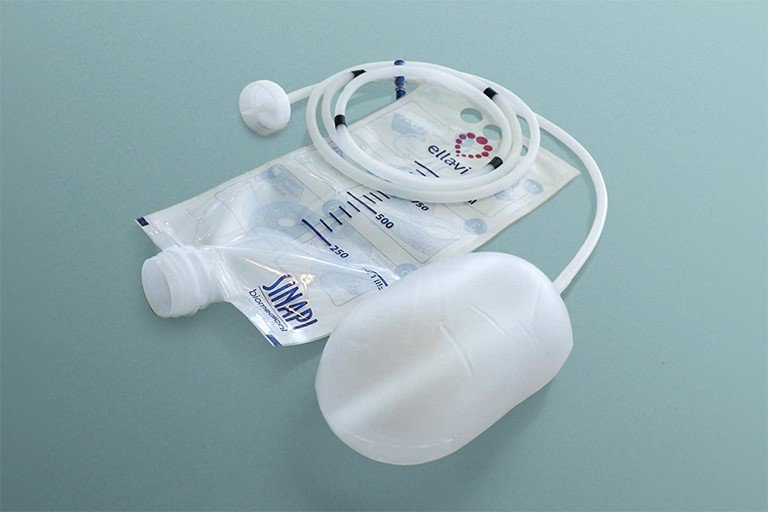
The Ellavi UBT is a gravity-filled open system designed to apply optimal pressure to stop postpartum bleeding fast. With widespread adoption, the device is projected to save 169,000 lives by 2030. Photo: PATH/Patrick McKern.
The Ellavi UBT costs around US$7.50 per unit—much lower than the current market price of UBTs in India. PATH is currently planning to introduce and scale distribution of this device across India. Our goal is to make this medically-regulated, affordable, lifesaving device available and accessible in every health care setting—and any woman in India experiencing postpartum hemorrhage.
2. Build evidence of impact
Organizations like PATH play an important role by “de-risking” new medical devices or technologies—that is, creating and sharing evidence of safety, efficacy, and potential health impact. Governments, health system managers, and patients want to know—based on hard evidence—that a product, drug, or approach actually works. Once that evidence exists, it’s easier to secure funding and commitments from key stakeholders.
For example, the World Health Organization has clear evidence about the role of pulse oximetry in treating severe illness in children. Working with health facilities in India to use pulse oximeters specifically designed for children under five, PATH is generating additional scientific evidence that can guide the expansion of these devices across the country. We believe the evidence will lead to wide-scale adoption.
3. Design for the user
Sometimes, even a great technology can fail to achieve scale because the design doesn’t meet the unique needs or constraints of thepeople who will use it.
User-centered design principles are gaining prominence as more people working on health product development understand the importance of including end users at every stage of the design process. PATH has long centered our design practices around patients and health workers—because how a product is designed doesn’t just determine how well it is used, but whether it is used at all.
We find it’s often the small or unexpected design choices that most influence a product’s adoption and ultimately, impact. Pulse oximeters offer one example of this phenomenon. Despite their proven utility in measuring the blood oxygen levels of adults and adolescents, pulse oximeters have not been widely adopted for newborns or young children—and no one knows precisely why.

By measuring pulse rate and oxygen saturation in the blood, a pulse oximeter provides objective patient information that can help health care workers identify severely ill children. Illustration: PATH/Thom Heileson.
Through the Unitaid-funded Tools for the Integrated Management of Childhood Illness (TIMCI) project, PATH is partnering with national governments across Africa and Asia to broaden the use of child-appropriate pulse oximeters. At the same time, we’re also and thinking about how to add additional features. A single tool that measures multiple vital statistics at once—like respiratory rate, temperature, hemoglobin level, and blood pressure—could streamline the diagnosis and monitoring of a host of childhood illnesses.
4. Collaborate across sectors
Introducing a new product or tool into a health system requires regulatory savvy, market dynamics expertise, strong supply chains, health worker trainings, and more. Governments, nonprofits, the private sector, regulatory bodies, and international organizations must work together to ensure the proper infrastructure is in place for a product to reach everyone who needs it.
PATH leverages existing partnerships—and regularly forges new ones across sectors and borders—to support the development, introduction, and wide distribution and adoption of appropriate health products like UBTs and pulse oximeters.
India has a robust and dynamic private sector capable of great innovation, but those novel products need public-sector support to reach scale. In India, and in countries around the world, PATH unites complementary partners from all sectors so that lifesaving products grow from idea to impact.
Four principles for a better future
Through TIMCI and other projects, PATH will continue applying these principles in India and in countries around the world. Together, with the help of our partners, we will continue increasing access, improving outcomes, and opening the door for future products that save lives and improve health for everyone.


